Summary
Previous trials comparing hypofractionation (HF) with conventional fractionation in whole breast irradiation (WBI) therapy have shown equal rates of local control, overall survival, and cosmesis. Notably, none of these trials included a tumor bed boost as part of the standard of care, although a tumor bed boost is known to decrease risk of locoregional recurrence and is widely used in the United States and Europe.
- Radiology
- Breast Cancer
- Radiation Therapy
- Oncology Clinical Trials
- Radiology
- Breast Cancer
- Oncology
- Radiation Therapy
- Oncology Clinical Trials
Previous trials comparing hypofractionation (HF) with conventional fractionation (CF) in whole breast irradiation (WBI) therapy have shown equal rates of local control, overall survival (OS), and cosmesis. Notably, none of these trials included a tumor bed boost as part of the standard of care, although a tumor bed boost is known to decrease risk of locoregional recurrence and is widely used in the United States and Europe.
Simona F. Shaitelman, MD, MEd, University of Texas MD Anderson Cancer Center, Houston, Texas, USA, described results at 6 months from an ongoing randomized trial (MD Anderson Protocol 2010-0559) comparing the impact of HF-WBI with that of CF-WBI (both including a tumor bed boost) on patient-reported cosmetic outcome at 3 years. Secondary objectives are determination of maximal skin and soft tissue toxicities arising from treatment and comparison of patient quality of life.
The protocol employed for CF-WBI was 50 Gy in 25 fractions over a period of 30 to 32 days with a tumor bed boost ranging from 10 to 14 Gy, whereas HF-WBI was administered at 42.56 Gy in 16 fractions over 20 to 21 days with a 10- to 12.5-Gy tumor bed boost. Standardi zed templates were used to collect data on acute toxicities using Common Terminology Criteria for Adverse Events (CTCAE) v4.0 criteria. In examining short-term (6-month) toxicities, the Late Effects in Normal Tissues Subjective, Objective, Management, and Analytic Scales and the Functional Assessment of Cancer Therapy-Breast (FACT-B) criteria were used in addition to CTCAE v4.0.
Eligibility required stage Tis to T2, N0 to N1, and M0 breast cancer. Patients were excluded if they had any prior history of breast cancer, concurrent bilateral breast cancer, history of prior radiotherapy (RT) to areas of potential overlap, or were pregnant. A total of 287 patients were enrolled and randomized, with 138 receiving HF-WBI. About 75% of patients were white (non-Hispanic), aged 50 to 70 years, had invasive cancers, and were either overweight or obese. Nearly 90% of patients were postmenopausal. About 15% of patients had grade 3 tumors, most had T1, N0 disease, and the majority had hormone receptor-positive, human epidermal growth factor 2-negative tumors. About 10% of patients had received neoadjuvant chemotherapy.
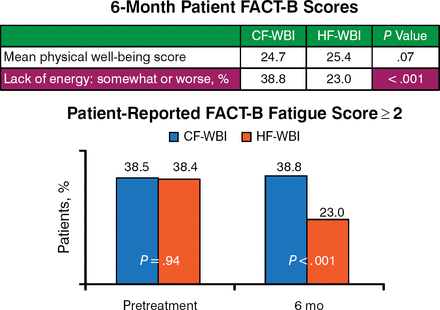
Baseline FACT-B mean scores for physical well-being and level of energy were essentially identical between the 2 groups. At 6 months after RT, physical well-being scores showed a trend of being slightly worse among patients treated with CF-WBI (P = .07). The percentage of patients reporting lack of energy “somewhat or worse” at 6 months was much higher in the CF-WBI group (38.3% to 23.0% for the HF-WBI group) and was statistically significant (P < .001; Figure 1).
Fatigue Reported by Hypofractionation and Conventional Fractionation Groups
CF, conventional fractionation; FACT-B, Functional Assessment of Cancer Therapy-Breast; HF, hypofractionation; WBI, whole breast irradiation.
Reproduced with permission from SF Shaitelman, MD, MEd.
Acute grade ≥ 2 toxicity was recorded weekly during RT and again at 6 months (Table 1). During weekly reports, 46.4% of HF-WBI patients had any acute grade ≥ 2 toxicity, compared with 77.9% for CF-WBI (P < .001), and HF-WBI patients had no acute grade ≥ 3 toxicities, compared with 5.4% for CF-WBI (P = .006). Although acute toxicity reporting at 6 months showed HF-WBI to be lower in 4 of 6 categories, the data were less clear-cut, with only fatigue data being statistically significant. Cosmesis data per se were not reported, because these are interim data sets (6 months), although several categories of acute toxicity could be used to infer aspects of cosmetic appearance.
WBI Toxicity Grade ≥ 2 Reported at 6 Months
Dr Shaitelman concluded that by the end of RT, the HF-WBI patients had less acute toxicity than those who had received CF-WBI. She added that the HF-WBI patient scores for patient-reported and physician-reported rates of fatigue 6 months after completing radiation were lower than those for CF-WBI and trended toward improved physical well-being in the HF-WBI arm.
- © 2014 MD Conference Express®