Summary
Early administration of eplerenone added to standard treatment in postmyocardial infarction patients appears to reduce the risk of adverse cardiovascular outcomes and heart failure, according to results from the Impact of Eplerenone on Cardiovascular Outcomes in Patients Post-Myocardial Infarction trial [REMINDER; NCT01176968].
- Heart Failure
- Cardiology Clinical Trials
- Myocardial Infarction
- Heart Failure
- Cardiology Clinical Trials
- Myocardial Infarction
- Cardiology
Early administration of eplerenone added to standard treatment in postmyocardial infarction (MI) patients appears to reduce the risk of adverse cardiovascular (CV) outcomes and heart failure (HF), according to results from the Impact of Eplerenone on Cardiovascular Outcomes in Patients Post-Myocardial Infarction trial [REMINDER; NCT01176968].
The randomized double-blind REMINDER trial included 1102 patients with ST-segment elevation myocardial infarction (STEMI) in the absence of HF. The lead investigator for the study, Gilles Montalescot, MD, PhD, Pitié-Salpétrière Hospital, Paris, France, reported that the provision of a mineralocorticoid receptor antagonist early in the acute phase of MI suggests a potential long-term CV benefit.
Eligible patients were identified following emergency room or ambulance evaluation and diagnosis of acute STEMI without HF. Key exclusion criteria were a known left ventricular ejection fraction (LVEF) <40%, any previous known history of HF, uncontrolled hypotension (systolic blood pressure <90 mm Hg), and known renal insufficiency (eGFR ≤30 mL/min/1.73m2). Patients were randomized to eplerenone (25 to 50 mg QD; n=506) or placebo (n=506), with the first dose of the study drug administered within 24 hours of symptom onset, and if possible, within 12 hours. All patients otherwise were to receive standard medical therapy.
The primary endpoint was a composite of CV mortality, rehospitalization or extended initial hospital stay due to HF, sustained ventricular tachycardia or ventricular fibrillation, LVEF ≤40% after 1 month post randomization, or elevated brain natriuretic peptide (BNP) or N-terminal pro-BNP (NT-proBNP) at 1 month post randomization.
Baseline characteristics were well balanced between the 2 treatment arms; the mean age of participants was 58 years, 17% were female, and 35% had anterior STEMI on presentation. Patient background medical therapy was representative of guideline-recommended practice (approximately 98% received aspirin, 98% P2Y12 antagonist, 79% heparins or fondaparinux, 29% a GPIIb/IIIa inhibitor); approximately 85% received primary percutaneous coronary intervention.
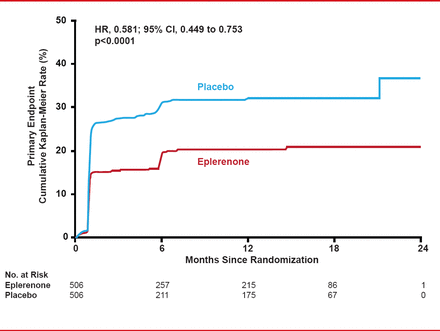
After a mean follow-up of 10.5 months, the primary composite endpoint was significantly lower in the eplerenone (18.4%) versus placebo group (29.6%; HR, 0.58; 95% CI, 0.45 to 0.75; p<0.0001; Figure 1). However, the main driver and only statistically significant component of the primary composite endpoint was the biochemical endpoint of elevated BNP/NT-proBNP (16.0% with eplerenone vs 25.9% with placebo; HR, 0.58; 95% CI, 0.44 to 0.77; p=0.0002).
Primary Composite Endpoint for Eplerenone Versus Placebo
Reproduced with permission from G Montelescot, MD, PhD.
Consistent with the primary endpoint were trends towards reductions in individual clinical events including CV mortality (HR, 0.52; 95% CI, 0.05 to 5.99; p=0.60) and HF rehospitalization or extended stay (HR, 0.56; 95% CI, 0.20 to 1.54; p=0.26). In addition, the trial was originally designed with an expected placebo-group primary event rate of 42%. Despite extending the sample size to accommodate for an observed lower than expected blinded aggregate event rate (21% at 6 months), the trial remained underpowered for the primary analysis.
Adverse events were balanced between groups with the exception of hyperkalemia (>5.5 mmol/L), which was more frequent with eplerenone (5.6% vs 3.2%; p=0.09).
REMINDER is the first study to demonstrate benefit and safety of early eplerenone treatment in patients presenting with STEMI in the absence of HF. The benefit was largely driven by biochemical improvements (BNP and NT-proBNP) but with consistent numerical reductions in clinical endpoints. Additional well-powered trials studying clinical outcomes would confirm these benefits.
- © 2013 MD Conference Express®