Summary
The last 3 decades have seen major advances in cardiovascular medicine and surgery. Unfortunately, the results of efforts to improve outcomes in patients with heart failure (HF) are mixed. Although patients with chronic HF with reduced ejection fraction (HFrEF) have benefited in terms of survival and quality of life from neurohormonal blockers and devices, there has been no improvement in the management of patients with HF with preserved EF, and little improvement in patients presenting with acute HF. This article summarizes the progress that has been made in the treatment of HF in six areas: biomarkers, microRNAs, calcium cycling, gene therapy, cell therapy, and left ventricular assist devices.
- Cardiology Genomics
- Heart Failure
- Cardiology Genomics
- Heart Failure
- Cardiology
The last 3 decades have seen major advances in cardiovascular medicine and surgery. Unfortunately, the results of efforts to improve outcomes in patients with heart failure (HF) are mixed. Although patients with chronic HF with reduced ejection fraction (HFrEF) have benefited in terms of survival and quality of life from neurohormonal blockers and devices, there has been no improvement in the management of patients with HF with preserved EF (HFpHF), and little improvement in patients presenting with acute HF. During the inaugural Braunwald Lecture, Eugene Braunwald, MD, Harvard Medical School, Brigham and Women's Hospital Boston, Massachusetts, USA, summarized the progress that has been made in the treatment of HF. He focused on six areas: biomarkers, microRNAs (miRNAs), calcium cycling, gene therapy, cell therapy, and left ventricular assist devices (LVADs).
BIOMARKERS
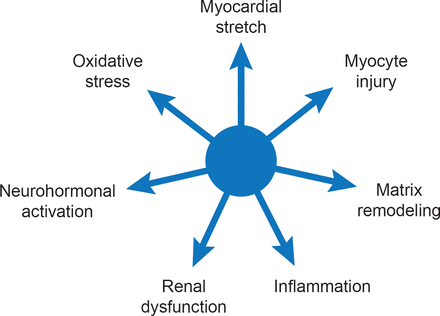
Currently available biomarkers for HF reflect seven biological processes that are independently associated with negative outcome (Figure 1) [Braunwald E. JACC Heart Fail 2013]. Several multimarker scoring systems have been developed to predict outcomes in different populations such as chronic HFrEF [Ky B et al. Circ Heart Fail 2012], HFpEF [Zile MR et al. Circ Heart Fail 2011] and patients without HF [Wang TJ et al. Circulation 2012]. Although the clinical utility of biomarker-guided therapy has been controversial, a recent meta-analysis (2700 patients; 12 trials) reported a significant (p=0.005) 26% mortality reduction among HF patients when natriuretic peptide-guided therapy was added to optimized medical therapy [Savarese G et al. PLoS One 2013].
Biomarker Profile of Heart Failure
Reproduced from Braunwald E. State-of-the-Art Paper: Heart Failure. JACC: Heart Failure 2013;1(1)1–20. With permission from Elsevier.
Dr. Braunwald sees multiple applications for biomarkers in HF including diagnosis, prognosis, risk assessment, as therapeutic targets, and potentially, as part of personalized therapy.
MICRORNAS
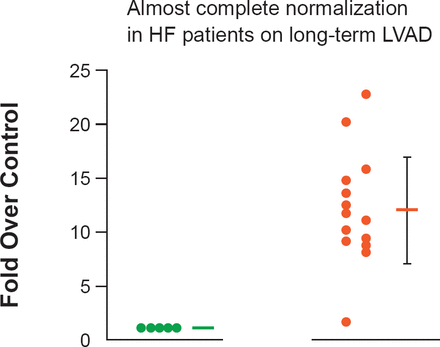
MiRNAs are short noncoding RNAs, which are present in all forms of life. More than 1600 have been isolated in humans. In HF, they have been shown to be associated with the development of hypertrophy (Figure 2) [Matkovich SJ et al. Circ Res 2012]. In the future they may have a role as biomarkers for HF [Tijsen AJ et al. Circ Res 2010], or as targets for the development of novel therapies [Krützfeldt J et al. Nature 2005; Wahlquist C et al. Nature 2014].
Expression of MiR-499 in Humans
HF=heart failure; LVAD=left ventricular assist devices.
Reproduced from Matkovich SJ et al. Direct and indirect involvement of microRNA-499 in clinical and experimental cardiomyopathy. Circ Res 2012 Aug 17;111(5):521–31. With permission from Lippincott Williams and Wilkins.
Dr. Braunwald believes that of all the new HF research areas, miRNAs hold the most promise by helping to improve our understanding of HF and its diagnosis, and ultimately in developing new therapies in antagonists to miRNAs (antagomirs).
CALCIUM CYCLING
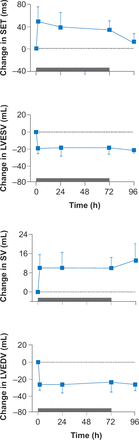
Calcium cycling (CA2+ cycling) is critical to normal cardiac contraction and relaxation. Disturbances in this process such as calcium leakage, insufficient release of calcium, or calcium overload are important factors in the development of HF [Luo M, Anderson ME. Circ Res 2013]. Medications that improve myocardial contractility and ventricular dysfunction hold promise for patients with HF. In a Phase 2 randomized, placebo-controlled clinical trial, the cardiac myosin activator, omecamtiv mecarbil, was shown to improve cardiac function in patients with HFrEF by increasing the sensitivity of cardiac myocytes to calcium (Figure 3) [Cleland JG et al. Lancet 2011]. Omecamtiv mecarbil is in Phase 3 trials.
Cardiac Myosin Activation in Patients With HFrEF
HFrEF=heart failure with reduced ejection fraction; LVEDV=left ventricular end-diastolic volume; LVESV=left ventricular end-systolic volume; SET= systolic ejection time; SV=systolic volume.
Reproduced from Cleland 1G et al. The effects of the cardiac myosin activator, omecamtiv mecarbil, on cardiac function in systolic heart failure: a double-blind, placebo-controlled, crossover, dose-ranging phase 2 trial. Lancet 2011; 378(9792):676–683. With permission from Elsevier.
GENE THERAPY
Despite difficult beginnings, much progress has been made with gene therapy in patients with HF over the last few years. Investigators have successfully introduced the SERCA2a gene into SERCA2a-ablated mice as well as in rat, pig, and sheep models of LV overload, and into isolated cardiomyocytes obtained from patients with HFrEF [Hajjar R et al. J Clin Invest 2013].
Gene therapy using adeno-associated virus type 1/sarcoplasmic reticulum Ca(2+)-ATPase was studied in the Phase 2 Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease trial [CUPID; Jessup M et al. Circulation 2011]. The treatment was well tolerated and led to improvements in clinical outcomes, symptoms, functional status, biomarkers, and cardiac structure. Gene expression persisted up to 31 months, with clinical benefits lasting up to 3 years [Zsebo K et al. Circ Res 2014]. Other potential gene targets include phospholamban and S100A1.
CELL THERAPY
One of the most challenging approaches to the treatment of HF is cell therapy. In addition to autologous cardiac cells, a broad range of other cell sources have been studied including bone marrow cells (BMCs), skeletal myoblasts, adipose-derived stem cells, hematopoietic stem cells, mesenchymal stem cells, and blood-derived endothelial progenitor cells. After cell implantation, myocardial repair is accomplished by activation of endogenous progenitor cells, inhibition of apoptosis, extracellular matrix remodeling, or the promotion of neovascularization [Sanganalmath SK, Bolli R. Circ Res 2013].
Cell therapy was studied in the Intracoronary Progenitor Cells in Acute Myocardial Infarction trial [REPAIR-AMI], which enrolled 204 patients with left ventricular dysfunction (LVD) or HF post-myocardial infarction (MI). Patients receiving intracoronary BMC had significant improvement in both EF (p=0.009) and wall thickening (p<0.001) compared with those receiving placebo at 2 years [Assmus B et al. Circ HF 2010]. Results of a meta-analysis of adult BMC therapy (50 studies; 2625 patients, most with LVD or HF post MI), indicated that BMC transplantation significantly improved survival, LV function, infarct size, and LV remodeling in patients with ischemic heart disease compared with standard therapy [Jeevanantham V et al. Circulation 2012].
In a Phase I study, carried out in patients with post-Mi HF who were enrolled into the Cardiosphere-Derived Autologous Stem Cells to Reverse Ventricular Dysfunction trial [CADUCEUS; Malliaras K et al. J Am Coll Cardiol 2014] and received autologous cardiac-derived cells (n=17) were noted to have a marked reduction in scar size, increased circumferential strain, and greater LV wall thickening of the infarcted section than in patients receiving placebo.
The first relatively large scale trial (n=3000) of cell therapy, the Effect of Intracoronary Reinfusion of Bone Marrow-Derived Mononuclear Cells (BM-MNC) on All-Cause Mortality in Acute Myocardial Infarction [BAMI; NCT01569178], is ongoing in 11 European countries. The objective of this Phase 3 trial, which is being conducted in patients who have a reduced LVEF (≤45%) following an AMI, is to demonstrate that BMC therapy is safe and reduces all-cause mortality compared with controls who are receiving optimal medical care.
Dr. Braunwald believes that cell therapy will play a role in the war against HF, but not alone. He sees this therapy being most useful when used in combination with LVADs.
LEFT VENTRICULAR ASSIST DEVICES
Newer continuous flow LVADs are smaller, have no mechanical bearings, and can generate outputs as high as 10 L/minute [Aaronson KD et al. Circulation 2012]. Although originally used as a bridge to transplant, there has been a steady increase in the use of LVADs as destination therapy. Survival rates with continuous flow devices are better than with the pulsatile devices [Kirklin JK et al. J Heart Lung Transplant 2013] but complications of prolonged LVAD use, such as infection and bleeding, remain a challenge.
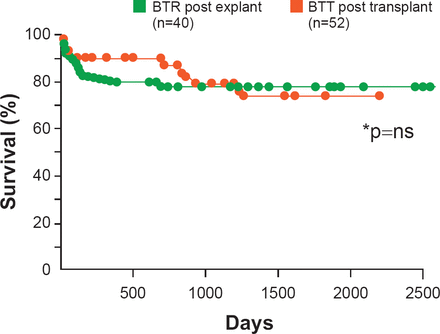
There is good news, however, in that reverse remodeling of the heart with continuous use of LVADs has been reported. There are reductions in circulating neurohormones and regression of cardiomyocyte hypertrophy, increases in myocardial contractility and in the density of beta adrenergic receptors, as well as improvements in CA2+ cycling [Ambardekar AV, Buttrick PM. Circ Heart Fail 2011]. These reports have led to attempts to wean patients from their assist devices. In one observational study, long-term survival in these patients who were able to be weaned off of a LVAD was similar when compared with patients who underwent heart transplant (Figure 4) [Birks EJ et al. J Thorac Cardiovasc Surg 2012]
Survival: Post Explantation/Transplantation
BTR=bridge to recovery; BTT=bridge to transplant.
Reproduced from Birks EJ et al. Long-term outcomes of patients bridged to recovery versus patients bridged to transplantation. J Thorac Cardiovasc Surg 2012; 144(1):190–196. With permission from Elsevier.
We have not yet seen remission of HF in patients with chronic ischemic cardiomyopathy, however. To achieve this Dr. Braunwald suggests considering earlier intervention (perhaps at Stage III) with LVAD in these patients and eliminating the use of transcutaneous lines to reduce infection.
In conclusion, Dr. Braunwald is optimistic that novel therapies offer the potential for progress in the war on HF.
- © 2014 MD Conference Express®