Summary
This article reviews several recent studies using focused ultrasound to facilitate treatment of movement disorders and commented on its future. Also discussed are recent Phase 3 studies that assessed disease modification in patients with Parkinson's disease (PD), novel technologies to understand the role of circuits and cells in animal models of movement disorders, and the role of leucine-rich repeat kinase 2 (LRRK2), PTEN-induced putative kinase 1 (PINK1), and parkin as genetic risk factors in sporadic PD.
- Extrapyramidal & Movement Disorders Clinical Trials
- Extrapyramidal & Movement Disorders
- Neurology Clinical Trials
- Neurology
THE USE OF FOCUSED ULTRASOUND IN THE TREATMENT OF MOVEMENT DISORDERS
Focused ultrasound is an early-stage, noninvasive therapeutic technology that uses ultrasonic energy to target tissue deep in the body without incisions or radiation. With this approach, magnetic resonance (MR) or ultrasound imaging is used to identify and target the tissue to be treated, to guide and control the treatment in real time, and to confirm the effectiveness of the treatment. Treatment is accomplished with an acoustic lens that concentrates multiple intersecting beams of ultrasound on a target with extreme precision and accuracy. Focused ultrasound treatments can be performed on an outpatient basis, require no incisions, and have the advantage of minimal discomfort and few complications, allowing rapid recovery.
José Obeso, MD, University of Navarra, Pamplona, Spain, reviewed several recent studies using focused ultrasound to facilitate treatment of movement disorders and commented on its future.
In one study, MR-guided focused ultrasound (MRgFUS) was used to ablate tremor-mediating areas of the thalamus in 4 patients with chronic, medication-resistant essential tremor [Lipsman N et al. Lancet Neurol 2013]. All 4 showed immediate and sustained improvements in tremor in the dominant hand (mean reductions in tremor score of 89.4% at 1 month and 81.3% at 3 months), which was accompanied by functional benefits and improvements in writing and motor tasks. One patient had postoperative paresthesia, which persisted at 3 months. Another developed a deep vein thrombosis, potentially related to procedure length. Similar improvements were noted in a Phase 1 study of 15 patients also with severe, medication-resistant essential tremor (mean age, 66.6 years; mean duration of tremor, 32.0±21.3 years), with a 75% improvement in hand tremor score, a 56% improvement in total tremor score, and an 85% reduction in disability by 12 months (Table 1) after treatment with MRgFUS [Elias WJ et al. N Engl J Med 2013]. Four patients had 100% improvement. Adverse events included transient sensory, cerebellar, motor, and speech abnormalities, with persistent paresthesia in 4 patients (2 in the lip or tongue, 1 in the finger).
Twelve-Month Treatment Resultsa
In the most recently published study, Chang and colleagues reported the results of MRgFUS thalamotomy in 11 patients with medication-resistant essential tremor [Chang WS et al. J Neurol Neurosurg Psychiatry 2014]. Three subjects could not complete the treatment because of insufficient temperature. Of the 8 who did complete the treatment, all showed immediate tremor reductions that were sustained at the 6-month follow-up. There were no significant postsurgical complications; about 50% of patients reported dizziness during the treatment. One Phase 3 multicenter, randomized sham-controlled trial [NCT01827904] is currently recruiting; results are expected in 2015.
According to Prof. Obeso, the clinical effects of focused ultrasound technology are similar to those of ablative and thermolytic lesioning, and as with the ablative procedures, the major limitations are the impact of the lesion itself and restriction of the lesion to one hemisphere. The main advantages are that there is no need for surgery and the possibility of earlier treatment (eg, in patients with Parkinson's disease [PD]). Technical issues such as how to achieve the correct size and precise location of the lesions and how to monitor clinical effects need further experience and development.
DISEASE MODIFICATION IN PARKINSON'S DISEASE
PD affects nearly 1 million Americans, and that number is expected to increase as the population ages. Symptoms of PD may include tremor, rigidity or stiffness of the limbs and trunk, slowness of movement, and impaired balance and coordination.
Karl Kieburtz, MD, MPD, University of Rochester, Rochester, New York, USA, discussed 2 recent Phase 3 studies that assessed disease modification in patients with PD, which were both stopped early for futility.
Creatine is a dietary supplement thought to improve exercise performance. Results of a Phase 2 futility clinical trial in PD [National Institute of Neurological Disorders and Stroke (NINDS) Neuroprotective Exploratory Trials in Parkinson Disease (NET-PD) Investigators. Clin Neuropharmacol 2008] indicated that creatine should be considered for a Phase 3 trial to determine whether it could alter long-term disease progression. The NET-PD Long-Term Study 1 (LS-1) Creatine in Parkinson's Disease trial [Elm JJ et al. Mov Disord 2012] was a Phase 3, multicenter, double-blind, placebo-controlled study to assess whether creatine monohydrate (5 g twice daily for 5 years) can slow disease progression in patients with early (within 5 years of diagnosis) PD treated with dopaminergic therapy. The primary outcome measure was change in clinical decline from baseline to 5-year follow-up on the basis of multiple outcome measures:
-
▪ Modified Schwab and England Activities of Daily Living Scale
-
▪ Parkinson's Disease Quality of Life Scale Summary Index
-
▪ Unified Parkinson's Disease Rating Scale Ambulatory Capacity
-
▪ Symbol Digit Modality Test
-
▪ Modified Rankin Scale
A total of 1741 subjects were enrolled and were to be followed for a maximum of 8 years, but during a planned interim analysis, the study was stopped for futility. At that time, 5-year results were available for 955 subjects (478 randomly assigned to placebo, 477 randomly assigned to creatine). Creatine was generally well tolerated and did not negatively affect renal function or weight gain. Within the context of this trial, however, creatine could not be recommended to slow disease progression in patients with PD.
Studies assessing the potential of coenzyme Q10 (CoQ10) followed a pattern similar to creatine. CoQ10 is an antioxidant that supports mitochondrial function and has been shown in preclinical PD models to reduce the loss of dopamine neurons. Early studies found CoQ10 to be safe and well tolerated at dosages of ≤1200 mg/day and to slow the progressive deterioration of function in PD [NINDS NET-PD Investigators. Neurology 2007; Shults CW et al. Arch Neurol 2002], but the Phase 3 trial failed to identify a clinical benefit [Parkinson Study Group QE3 Investigators. JAMA Neurol 2014].
Dr. Kieburtz suggested that the problem with both programs was not the design and conduct of the Phase 3 study but likely the choices made in Phase 2 concerning which compound to move forward.
NOVEL TECHNOLOGIES TO CLARIFY THE NEURAL CONNECTIONS IN ANIMAL MODELS OF MOVEMENT DISORDERS
A connectome is a comprehensive map of neural connections in the brain. The human connectome is extremely complex, with different regions often connecting with functionally distinct classes of neurons within the same region or nucleus. D. James Surmeier, PhD, Northwestern University, Feinberg School of Medicine, Chicago, Illinois, USA, discussed some of the novel technologies that will allow scientists to better understand and capitalize on the role of circuits and cells in animal models of movement disorders.
The monosynaptic recombinant rabies virus (mrRV) offers a way of identifying cells that are monosynaptically connected to a population of interest as well as a way to transsynaptically label cells connected to a single starting cell [Wickersham IR et al. Neuron 2007]. The rabies virus in the mrRV is modified by replacing the coat glycoprotein with a fluorescent reporter and adding an envA expression construct to restrict binding to a TVA receptor only. Infection with mrRV is limited to neurons expressing the virally introduced TVA receptor. The spread is retrograde only, because mrRV is nonreplicating and monosynaptic. The infection is relatively benign within an approximately 2-week observation window. The fluorescent reporter expression is very robust.
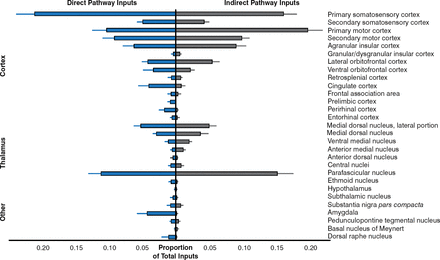
BAC-Cre mouse lines are widely available for many populations of neurons of the brain and provide a means of expressing the TVA receptor that allows cells to be infected by mrRV [Gerfen CR et al. Neuron 2013]. A recent study that used mrRV to map inputs to generate brainwide maps of neurons that form synapses with direct- or indirect-pathway striatal projection neurons showed that sensory cortical and limbic structures preferentially innervated the direct pathway, whereas motor cortex preferentially targeted the indirect pathway (Figure 1) [Wall NR et al. Neuron 2013].
Pathway Preferences
Reproduced from Wall NR, De La Parra M, Callaway EM, Kreitzer AC. Differential innervation of direct- and indirect-pathway striatal projection neurons. Neuron 2013;79:347–360. With permission from Elsevier.
Designer receptors exclusively activated by designer drugs (DREADDs) are powerful tools for the study of G-protein-coupled receptor (GPCR) signaling and physiology [Wess J et al. Trends Pharmacol Sci 2013]. They are modified GPCRs that can be activated only by clozapine-N-oxide (CNO), which can be administered systemically and has no discernible off-target effects. DREADDs that couple with all of the major signaling pathways are available. Cre-dependent and independent DREADD expression constructs can be delivered using viral vectors [Lee HM et al. Drug Disc Today 2014].
The 2 most commonly used DREADDs are muscarinic receptors in which the binding pocket has been reengineered so that it binds only to CNO. The hM3Dq DREADD couples to the Gq/11 pathway and typically leads to neuronal excitation, while the hM4Di DREADD, which is a Gi/o-coupled receptor, typically leads to activation of potassium channels and inhibition of neurons [Wess J et al. Trends Pharmacol Sci 2013]. These receptors provide a way to “turn up” or “turn down” excitability in cells while allowing them to continue to respond to their normal synaptic input. Using BAC-Cre lines, viral DREADD expression can be limited to a particular cell-type in a particular region.
In a mouse model of PD, the intrinsic activity of subthalamic neurons is down-regulated, thus contributing to network pathology. Dr. Surmeier discussed as yet unpublished studies in which Bevan and colleagues have found that this deficit can be corrected with a virally delivered Gq-DREADD. They have also shown that DREADD activation dramatically reduces forelimb asymmetry in unilaterally 6-OHDA lesioned mice.
In Dr. Surmeier's opinion, both mrRV and DREADDs are very exciting techniques that give us the ability not only to look at synaptic connections but also to manipulate the activity of very well-defined cell populations in the brain.
UNDERSTANDING THE ROLE OF LEUCINE-RICH REPEAT KINASE 2, PTEN-INDUCED PUTATIVE KINASE 1, AND PARKIN IN PD
Ryosuke Takahashi, MD, Kyoto University Hospital, Kyoto, Japan, discussed recent publications that have enhanced understanding of the role of leucine-rich repeat kinase 2 (LRRK2), PTEN-induced putative kinase 1 (PINK1), and parkin as genetic risk factors in sporadic PD.
In the first study, Imai and colleagues [EMBO J 2008] proposed that mutations in LRRK2 cause dopaminergic degeneration in patients with PD through phosphorylation of eukaryotic initiation factor 4E– binding protein (4E-BP). Chronic inactivation of 4E-BP by LRRK2 with pathogenic mutations deregulates protein translation, eventually resulting in age-dependent loss of dopaminergic neurons.
More recently, Martin and colleagues [Cell 2014] suggested that the phosphosubstrate that connects LRRK2 kinase activity to neurodegeneration is ribosomal protein s15. In that study, ribosomal protein s15 phosphorylation by LRRK2 was shown to stimulate both cap-dependent and internal ribosome entry site– mediated protein translation and, through an as yet unknown mechanism, to lead to neurodegeneration. Phosphomutant s15 rescued neurodegeneration in LRRK2 G2019S transgenic Drosophila.
Prof. Takahashi concluded that in a subset of LRRK2 mutation–related parkinsonism, translational dysregulation leading to overproduction of proteins may be causative and that a future therapeutic option for PD may be found in global translational repression.
In the second part of his presentation, Prof. Takahashi turned his attention to PINK1 and parkin, which are known to be responsible for the autosomal-recessive young-onset form of familial PD and essential for the selective elimination of damaged mitochondrion through the autophagy-lysosome pathway.
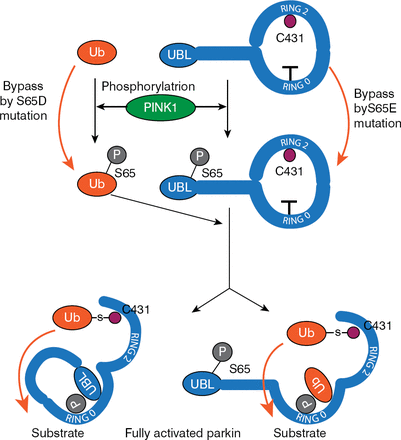
PINK1-dependent S65 phosphorylation is important for the activation of parkin [Shiba-Fukushima K et al. Sci Rep 2012]; however, it can only partially activate it. To fully activate parkin, an additional substrate is necessary. Ubiquitin is also phosphorylated by PINK1, and this phosphorylated ubiquitin is essential for parkin activation [Matsuda N et al. J Cell Biol 2010]. Koyano and colleagues [Nature 2014] have proposed a model for parkin activation that is based on interaction between ubiquitin and parkin by which PINK1-dependent phosphorylation of both is sufficient for full activation of parkin E3 activity (Figure 2).
A Model for Parkin Activation
P=parkin; PINK1=PTEN-induced putative kinase 1; Ub=ubiquitin.
Reproduced from Koyano F, Okatsu K, Kosako H, et al. Ubiquitin is phosphorylated by PINK1 to activate parkin. Nature 2014;510:162–166. With permission from the Nature Publishing Group.
In summary, noted Prof. Takahashi, PINK1 phosphorylates both ubiquitin and parkin, thus allowing phosphorylated ubiquitin to activate parkin by binding to and changing the conformation of phosphorylated parkin. Therefore, an excellent biomarker for mitochondrial damage may be phosphorylated ubiquitin.
- © 2014 MD Conference Express®