Summary
The FAER-Helrich Research Lecture focused on how neuroscience research is rapidly changing our understanding of anesthesia and how a deeper understanding of neuroscience can change patient care. Specific topics include the neural circuit mechanisms of commonly used anesthetics, electroencephalogram (EEG) signatures, how EEGs and their spectrograms can be used to track the state of anesthesia, how to control sedation, and how the brain dynamics under anesthesia change with age.
- Neuroimaging Neuroimaging
- General Anesthesia
- Neuroimaging
- General Anesthesia
Emery N. Brown, MD, PhD, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA, presented the American Society of Anesthesiologists' FAER–Helrich Research Lecture. Dr Brown's lecture focused on how neuroscience research is rapidly changing our understanding of anesthesia and how a deeper understanding of neuroscience can change patient care. During his presentation, Dr Brown reviewed the neural circuit mechanisms of commonly used anesthetics, electroencephalogram (EEG) signatures, how EEGs and their spectrograms can be used to track the state of anesthesia, how to control sedation, and how the brain dynamics under anesthesia change with age.
General anesthesia is defined as a drug–induced, reversible state composed of unconsciousness, amnesia, analgesia, akinesia, stability, and control. It is essential for performing most major surgical procedures. While under general anesthesia, patients experience several altered states of arousal: sedation unconsciousness, sedation analgesia, dissociative anesthesia, pharmacologic non–rapid eye movement sleep, and neuroleptic anesthesia. Each of these states is the result of the anesthetic drug acting at multiple targets in the central nervous system. Different drugs act at different sites [Brown EN et al. Annu Rev Neurosci. 2011] and can be tracked along the affected neural circuits. Understanding the relationship between this circuit and the affected body part can provide insight into the drug's action.
It is sometimes said that EEGs provide a “noisy” signal. Although this may be true, when used in environments like a sleep laboratory in which patients are moving about, EEGs can be used to clearly mark the progression from consciousness to unconsciousness in patients who are anesthetized.
EEG spectral signatures that track loss and recovery of consciousness under general anesthesia (eg, propofol) show a rise in frontal persistent and synchronous α activity at dose levels sufficient to induce loss of consciousness [Ching S et al. Proc Natl Acad Sci U S A. 2010]. By combining spectral and global coherence analyses, a new approach to tracking brain states under general anesthesia is proving to be valuable [Cimenser A et al. Proc Natl Acad Sci U S A. 2011].
Using propofol as an example, Dr Brown offered the following explanation of how anesthesia produces unconsciousness. Propofol acts at presynaptic and postsynaptic GABAA receptors within GABAergic synapses creating oscillations including α (8–12 Hz) rhythms that strongly couple the thalamus and cortex areas restricting communication, slow–wave (< 1 Hz) rhythms that create local islands preventing local cortex communication, and anteriorization, which acts as a mechanism for frontal–parietal disconnection [Purdon PL et al. Proc Natl Acad Sci U S A. 2013]. Communication between the lower parts of the brain and the cortex is impeded by blocking the brain stem arousal pathways. Different anesthetics act on different systems and have different EEG signatures and power spectrum structures. Knowledge of these neural circuits and individual drug signatures is important for understanding how and where the anesthesia is working.
The development state of the brain affects how anesthesia will work. Total EEG power peaks between 4 and 8 years of age, and then begins to drop off. Spatiotemporal spectrogram analysis shows a difference in EEG wave development between infants aged 0 to 3 months and those aged 4 to 6 months, with slow waves being more prominent in the younger infants.
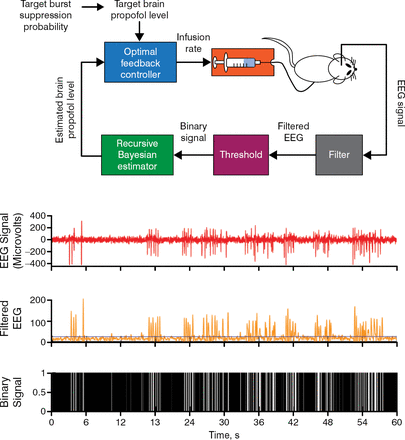
Burst suppression is a state of brain inactivation in which bursts of electrical activity alternate with periods of quiescence or suppression. It is seen in patients under general anesthesia, individuals with hypothermia, and among infants with early infantile epileptic encephalopathy or refractory seizures, each of whom demonstrate a clear pattern. Burst suppression is also seen in patients in a coma and can be manipulated to achieve a medically induced coma to treat various conditions or to protect the brain from further injury. One way to induce a coma is by manually titrating and maintaining the anesthesia infusion rate to a specified level. Medical personnel currently rely on visually monitoring the EEG to maintain appropriate levels of burst suppression. A promising alternative is the use of a brain–machine interface device for maintenance. One such device has been shown to maintain precise target levels of burst suppression by monitoring an EEG–guided closed–loop infusion of the anesthetic. In an animal model of medically induced coma using propofol, a brain–machine interface device accurately controlled burst suppression with a median performance error of 3.6%, and a median bias of −1.4% and overall posterior probability of reliable control of 1 was observed (95% Bayesian credibility interval, 0.87 to 1.0; Figure 1) [Shanechi MM et al. PLoS Comput Biol. 2013].
A Brain–Machine Interface Approach to Control Burst Suppression and Coma Level in a Rat Model
EEG, electroencephalogram.
Reprinted from Shanechi MM et al. A brain–machine interface for control of medically–induced coma. PLoS Comput Biol, 2013; DOI: 10.1371/journal.pcbi.1003284. Copyright: © 2013 Shanechi et al.
For most patients, emergence from anesthesia is a passive event; however, at times it may be necessary or even beneficial to actively speed/manage this process. A number of drugs have been used to achieve this. Results from a recent study in rats under general anesthesia with isoflurane or propofol demonstrated that electrical stimulation of the ventral tegmental area can be used to induce a graded arousal response and a shift in EEG peak power that increased with current intensity [Solt K et al. Anesthesiology. 2014]. This may provide a novel, nondrug approach to hasten recovery from general anesthesia or to treat postemergence problems.
In conclusion, Dr Brown emphasized the complex nature of the state of general anesthesia and its many associations with other altered states of consciousness (eg, sleep, hibernation, meditation, and drug addiction). A better understanding of EEGs can be used to manage patients in the operating room. It is also possible to integrate molecular information with neural circuits, EEG dynamics (neurophysiology), and behavior. This requires that the anesthesiologists have a deeper neuroscience understanding of neuroanatomy and neurophysiology with an emphasis on neural circuits, the need for a clinical neurology examination, working knowledge of neurophysiology of the brain's EEG, and a good understanding of brain and central nervous system–based pharmacology.
- © 2014 MD Conference Express®