Summary
Although the definition of transient ischemic attack (TIA) was updated in 2009 [Easton JD et al. Stroke 2009], no formal update of the definition of stroke has been undertaken since 1980. This article discusses the major aspects of the American Heart Association (AHA)/American Stroke Association (ASA) Expert Consensus Document on stroke [Sacco RL et al. Stroke 2013]. The new document incorporates clinical and tissue criteria of importance to clinical practice, research, and public health assessments in a newly updated definition of stroke.
- Neurology Guidelines
- Ischemia
- Neurology Guidelines
- Neurology
- Ischemia
- Exclusive Article - For home page
Although the definition of transient ischemic attack (TIA) was updated in 2009 [Easton JD et al. Stroke 2009], no formal update of the definition of stroke has been undertaken since 1980. Scott E. Kasner, MD, University of Pennsylvania, Philadelphia, Pennsylvania, USA, discussed the major aspects of the American Heart Association (AHA)/American Stroke Association (ASA) Expert Consensus Document on stroke [Sacco RL et al. Stroke 2013]. The new document incorporates clinical and tissue criteria of importance to clinical practice, research, and public health assessments in a newly updated definition of stroke.
Consistent with the 1980 definition of stroke, the presence of global ischemia alone is not sufficient to qualify as stroke under the new definition. The revised definition of central nervous system (CNS) infarction has been harmonized with existing definitions as:
-
▪ Brain, spinal, or retinal cell death attributable to ischemia, based on pathological, imaging, or other objective evidence of cerebral, spinal cord, or retinal focal ischemic injury in a defined vascular distribution; OR
-
▪ Clinical evidence of cerebral, spinal cord, or retinal focal ischemic injury based on symptoms persisting ≥24 hours or until death, and other etiologies excluded.
Ischemic stroke is now defined as “an episode of neurological dysfunction caused by focal cerebral, spinal, or retinal infarction.”
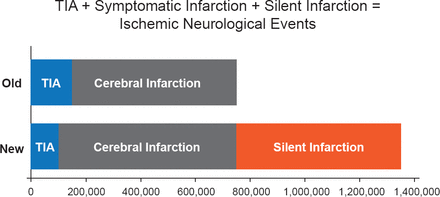
An important issue now specifically addressed in the Consensus Document is silent infarctions, which are defined as evidence of CNS infarction on imaging or with other neuropathologic evidence in individuals without a history of acute neurologic dysfunction attributable to the lesion. The definitional changes with respect to infarction mean that some prior TIAs will now be defined as stroke. The inclusion of silent infarctions, in particular, is expected to lead to a large increase in the prevalence of stroke as silent events are believed to significantly outnumber clinical events (Figure 1).
Net Increase in Stroke
TIA=transient ischemic attack.
With the new definition of stroke, the term hemorrhagic stroke, which previously included intracerebral and subarachnoid hemorrhage as well as hemorrhagic conversion of infarction, has been abandoned. It is replaced by definitions for each of its components.
The inclusion of silent disease means that stroke will become more common but at the same time less severe. From the perspective of clinicians, this indicates an expanded role for imaging and more emphasis on how to treat and evaluate this condition. Among researchers and public health experts, the challenge will be to further characterize the natural history of silent stroke. Moving forward, it is likely that both definitions will need to be used to make comparisons.
For decades, the standard therapy for atrial fibrillation (AF) has been warfarin. Although extremely effective at reducing the risk of cardio-embolic stroke, once-daily warfarin is also linked to multiple drug and dietary interactions, requires regular monitoring, and is associated with a major annual bleed rate of 1.3%. Karen Furie, MD, MPH, Massachusetts General Hospital, Boston, Massachusetts, USA, discussed dabigatran, rivaroxaban, and apixaban as replacements for warfarin and how they fit with the current AF treatment guidelines [Anderson JL et al. Circulation 2013; Furie KL et al. Stroke 2012].
The direct thrombin inhibitor dabigatran was the first therapy to be studied as a replacement for warfarin. Dabigatran has a half-life of 12 to 17 hours. It is dosed twice daily; excretion is via the kidney. Drug interactions are far fewer than with warfarin. The most important dabigatran trial was RE-LY [Connolly SJ et al. N Engl J Med 2009]. In this large trial, patients (n=18,113) with nonvalvular AF who received dabigatran 110 mg BID experienced rates of stroke and systemic embolism (SE) similar to patients on warfarin but with lower rates of major hemorrhage after 2 years of follow-up. At a dose of 150 mg BID, dabigatran was associated with lower rates of stroke and SE but rates of major hemorrhage that were similar to warfarin. Rivaroxaban is a once-daily direct Factor Xa inhibitor with a half-life of 5 to 9 hours. It has only limited drug interactions and is eliminated via the urine and feces. The primary rivaroxaban trial was ROCKET-AF [Patel MR et al. N Engl J Med 2011] which enrolled 14,264 patients with nonvalvular AF. Participants were randomized to rivaroxaban 20 mg QD or adjusted dose warfarin. After ∼2 years of follow-up, rivaroxaban was noninferior to warfarin on the primary outcome of stroke or SE. The difference in the risk of major bleeding was not significant; intracranial and fatal bleeding occurred less frequently in the rivaroxaban group. Apixaban, another Factor Xa inhibitor used in the treatment of nonvalvular AF, has a half-life of 8 to 15 hours and is dosed once daily. The route of excretion and drug interactions are similar to those of dabigatran. The ARISTOTLE study enrolled 18,201 patients [Granger CB et al. N Engl J Med 2011]. After 1.8 years of follow-up, apixaban 5 mg BID was superior to warfarin for preventing stroke and SE, caused less bleeding, and resulted in lower mortality.
In light of the data from these trials, all of these therapies are now recommended for the prevention of stroke in patients with non-valvular AF (Table 1) [Furie KL et al. Stroke 2012]. The safety and efficacy of combining dabigatran, rivaroxaban, or apixaban with an antiplatelet have not been established (Class IIb Level of Evidence [LOE] C).
Recommendations
Stroke is the third leading cause of death among women and the fifth among men [Centers for Disease Control. Women's Health 2010 http://www.cdc.gov/women/lcod/2010/index.htm; Men's Health 2010 http://www.cdc.gov/men/lcod/2010/index.htm]. Women have a 20% lifetime risk of stroke (vs 17% for men) [Seshadri S et al. Stroke 2006] and have poorer recovery and worse quality of life post stroke [Bushnell C et al. Neurology 2014]. Cheryl Bushnell, MD, MHS, Wake Forest University School of Medicine, Winston Salem, North Carolina, USA, discussed new guidelines [Bushnell C et al. Stroke 2014] for the prevention of stroke in women, which take into account the stroke risk factors that are either unique to (eg, pregnancy and its complications, the use of oral contraceptives [OCs], and hormonal treatments for menopause) or more common among women (eg, migraines with aura, hypertension, AF, and obesity/metabolic syndrome).
Since pregnancy, and preeclampsia in particular, increases the risk for hypertension and stroke later in life, the new guidelines recommend the following:
-
▪ Women with chronic primary or secondary hypertension or previous pregnancy-related hypertension take the daily low-dose aspirin beginning in the twelfth week of gestation until delivery (Class I LOE A)
-
▪ Oral calcium supplementation >1 g/day be considered for women whose daily calcium intake is <600 mg/day (Class I LOE A)
-
▪ Treatment of moderate hypertension (150 to 159/100 to 109 mm Hg) in pregnancy (Class IIa LOE B)
Other new recommendations are shown in Table 2.
Additional Recommendations to Reduce Stroke in Women
Dr. Bushnell suggested that development of a sex-specific risk score may be useful. Establishing a better understanding of the unique risks women face could help to encourage healthy lifestyles earlier in life.
- © 2014 MD Conference Express®