Summary
The PEGASUS-TIMI 54 trial demonstrated that continuing treatment with ticagrelor after 1 year following myocardial infarction protected patients from ischemic events and provided a more robust risk reduction compared to reinitiation in stable patients on aspirin monotherapy. Patients who were treated with only aspirin for > 1year after myocardial infarction did not derive any benefit from reinitiation of P2Y12 inhibitor therapy and had an increased risk of bleeding.
- cardiology & cardiovascular medicine clinical trials
- myocardial infarction
- MACE
- PEGASUS-TIMI 54
- P2Y12 inhibitors
- ticagrelor
- TIMI bleeding
The PEGASUS-TIMI 54 trial evaluated the efficacy and safety of ticagrelor, a P2Y12 receptor antagonist, in 21 162 patients who had a myocardial infarction (MI) event 1 to 3 years earlier. Patients were randomly assigned to treatment with ticagrelor 90 or 60 mg BID or placebo; all patients received aspirin. The primary efficacy end point was the composite of cardiovascular (CV) death, MI, or stroke at a median 33 months of follow-up. The primary safety end point was TIMI major bleeding. The investigators reported that both ticagrelor doses significantly reduced the rate of the composite end point compared with placebo [Bonaca MP et al. N Engl J Med. 2015].
Marc P. Bonaca, MD, Brigham and Women’s Hospital, Boston, Massachusetts, USA, presented the results of a subanalysis of the PEGASUS-TIMI 54 trial that assessed the effect of ticagrelor on reducing atherothrombotic events in post-MI patients, based on the time from withdrawal of their previous P2Y12 inhibitor therapy. The investigators hypothesized that patients withdrawn from P2Y12 inhibition at or shortly prior to randomization would have a relatively high ischemic risk compared with patients who had survived event free on aspirin therapy alone for a prolonged period and therefore would have a more robust reduction in ischemic risk with ticagrelor therapy.
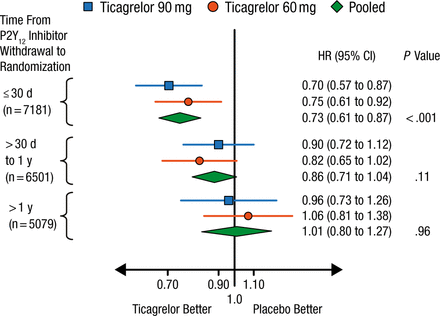
The patients were stratified at the time of randomization according to time from P2Y12 inhibitor withdrawal: ≤ 30 days (n = 7181), > 30 days to 1 year (n = 6501), and > 1 year (n = 5079). Patients taking placebo who recently stopped P2Y12 inhibition (≤ 30 days) had a higher risk for major adverse cardiac events (9.9%; HR, 1.47; 95% CI, 1.12 to 1.93), as did those who had stopped therapy 30 days to ≤ 1 year previously (8.7%; HR, 1.28; 95% CI, 0.98 to 1.67), compared with patients who had stopped therapy > 1 year previously (6.9%; Ptrend = .0097).
The benefit of ticagrelor treatment was greatest among patients randomized to ticagrelor within 30 days of P2Y12 inhibitor withdrawal even if MI was > 2 years ago, with a 27% risk reduction for CV death, myocardial infarction, or stroke (Figure 1). Patients who started ticagrelor > 30 days to 1 year from P2Y12 withdrawal had a 14% reduced risk, while those who started ticagrelor > 1 year after P2Y12 withdrawal derived no benefit.
Reduction in Major Adverse Cardiac Events With Ticagrelor by Time From P2Y12 Inhibitor Withdrawal
Reproduced with permission from MP Bonaca, MD.
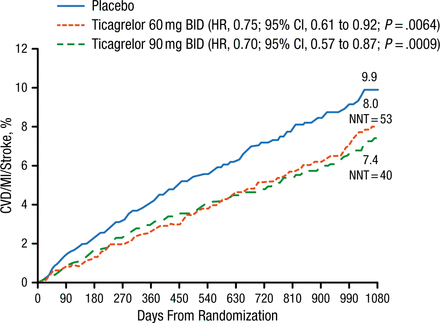
At 3 years, patients treated with ticagrelor within 30 days of randomization had a significantly lower risk of ischemic events vs those treated with placebo (ticagrelor 90 mg, P = .0009; ticagrelor 60 mg, P = .0064; Figure 2).
Major Adverse Cardiac Events With Ticagrelor in Patients With P2Y12 Inhibitor Withdrawal ≤ 30 Days From Randomization
CVD, cardiovascular death; MI, myocardial infarction; NNT, number needed to treat.
Reproduced with permission from MP Bonaca, MD.
The TIMI major bleeding rate was significantly higher with ticagrelor compared with placebo at 3 years.
These results showed that continuing P2Y12 inhibition beyond 1 year after MI provided a robust benefit for reducing cardiac events. Reinitiation of P2Y12 inhibition in patients who had survived without ischemic events on aspirin alone for > 1 year did not appear to provide any benefit and increased the risk of bleeding. Ongoing research using clinical, biochemical, and genetic factors may provide further prospective data for defining the optimal patient populations for long-term therapy.
- © 2015 SAGE Publications