Summary
The randomized phase 3 TNT trial, conducted to compare the efficacy of 2 chemotherapy drugs, carboplatin and docetaxel, at treating patients with advanced triple-negative breast cancer, showed that although survival outcomes were similar with both drugs, carboplatin was more efficacious in patients with BRCA1/2 gene mutations.
- estrogen receptor
- progesterone receptor
- HER2
- triple negative
- survival
- carboplatin
- docetaxel
- Triple Negative Breast Cancer Trial
- TNT
- NCT00532727
Andrew Tutt, MB, ChB, PhD, King’s College London, London, United Kingdom, shared results from the Triple Negative Breast Cancer Trial [TNT; NCT00532727], a randomized phase 3 trial of carboplatin compared with docetaxel in estrogen receptor (ER)–, progesterone receptor (PR)–, and human epidermal growth factor receptor 2 (HER2)–negative breast cancer (BC). Although the data showed no difference in survival among patients treated with either drug, carboplatin did provide benefit for those with BRCA1 or BRCA2 gene mutations.
Although triple-negative BC (TNBC) is a heterogeneous disease, distinct subgroups are associated with BRCA1/2 gene mutation, including immunohistochemical and gene expression basal-like subtypes [Sharma P et al. Breast Cancer Res Treat. 2014; Lehmann BD et al. J Clin Invest. 2011; Parker JS et al. J Clin Oncol. 2009; Cheang MCU et al. Clin Cancer Res. 2008]. However, although data have demonstrated sensitivity of some triple-negative and BRCA1/2 gene mutation–associated BCs to platinums [Isakoff SJ et al. J Clin Oncol. 2014; Telli ML et al. J Clin Oncol. 2013; Bryski T et al. Breast Cancer Res. 2012], studies have not compared single-agent platinum chemotherapy with standard of care and mechanistically distinct taxanes in these patient populations.
The TNT trial was conducted in women (n = 376) with metastatic or recurrent, locally advanced TNBC or BRCA1/2 gene mutation–associated BC. Patients were enrolled if they had ER-negative, PR-negative, and HER2-negative BC, or a known BRCA1/2 gene mutation. Exclusion criteria included adjuvant taxane therapy within the past 12 months, previous platinum therapy, or nonanthracyclines for metastatic BC. Participants were randomized 1:1 to receive either carboplatin (C; area under the curve 6) or docetaxel (D; 100 mg/m2) every 3 weeks for 6 cycles or until disease progression.
The primary end point was objective response rate (ORR). Secondary end points included progression-free survival (PFS), overall survival (OS), crossover treatment ORR, and toxicity. At a median follow-up of 11 months, ORR, median PFS, and median OS were similar in both treatment groups (Table 1).
TNT Results
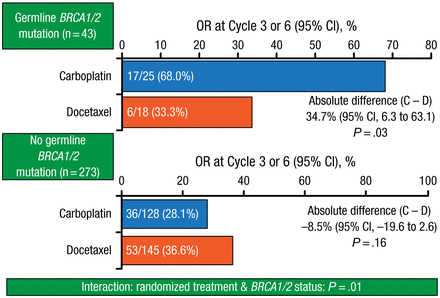
However, carboplatin therapy was associated with significantly improved ORR (68.0% vs 33.3%; P = .03; Figure 1) in patients with BRCA1/2 gene mutations, in whom median PFS was 6.8 months, compared with 3.1 months for mutation-negative patients.
Objective Response in Patients With and Without BRCA Gene Mutations
BRCA, breast cancer gene; C, carboplatin; D, docetaxel; OR, objective response.
Reproduced with permission from A Tutt, MB, ChB, PhD.
In the docetaxel group, median PFS was 4.8 and 4.6 months, respectively, among patients with and without BRCA1/2 gene mutations.
With respect to other disease subtypes, only patients with nonbasal-like disease responded differently to the 2 treatments, with a significantly increased ORR in the docetaxel group (16.7% vs 73.7%; absolute difference, –55.5%; 95% CI, –82.4 to −28.6; P < .01).
The safety data were as anticipated for both drugs. Carboplatin was generally better tolerated than docetaxel, with febrile neutropenia and neuropathy significantly more common in the docetaxel group (both P < .01).
Prof Tutt emphasized that the results of this trial support BRCA1/2 genotyping to guide therapy choice in advanced triple-negative and familial BC.
- © 2014 SAGE Publications