Summary
Atrial fibrillation is a common arrhythmia that presents management challenges to clinicians. Pulmonary vein isolation may help patients with paroxysmal and persistent AF convert to prolonged sinus rhythm. However, patients in need for additional substrate modification will likely require radiofrequency ablation as the preferred ablation technology. For patients with low left ventricular ejection fraction, cardiac resynchronization therapy may be achieved by simultaneously pacing both the left and right ventricles; endocardial pacing is likely superior to epicardial pacing.
- atrial fibrillation
- pulmonary vein isolation
- ablation
- cardiac resynchronization therapy
- cryoballoon
- contact force
- interventional techniques & devices
Atrial fibrillation (AF) is the most common heart rhythm abnormality in people aged > 65 years. Stasis caused by the lack of organized atrial contraction increases the risk of thrombus formation in the left atrium potentially resulting in embolic stroke. The estimated risk of stroke among all AF patients is 5% per year [Sellers MB, Newby LK. Am Heart J. 2011]. AF is the most common arrhythmia worldwide, with a reported prevalence of 1% to 2% [Camm AJ et al. Europace. 2010] and it is anticipated that the incidence will increase over the next several decades. One approach to treating AF in symptomatic patients is catheter-based ablation to try to restore and maintain normal sinus rhythm.
Erik Wissner, MD, Asklepios Klinik St Georg, Hamburg, Germany, spoke of challenges in developing novel catheters for isolating the pulmonary veins in patients with paroxysmal atrial fibrillation (PAF). It is well established that the electrical signals from the pulmonary veins initiate some forms of PAF [Haïssaguerre M et al. N Engl J Med. 1998]; however, isolation of the veins has proven challenging. Since 1998, point-by-point ablation has been used to create a circle around the ipsilateral pulmonary veins, but the data on long-term clinical outcomes have been limited.
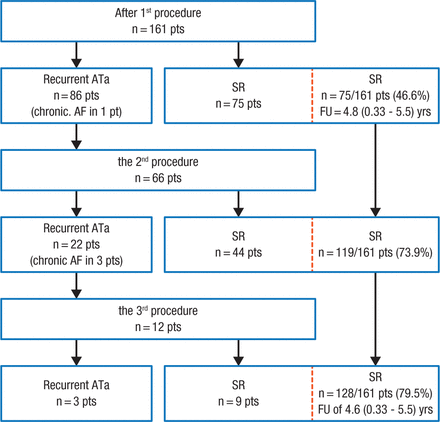
Dr Wissner highlighted data from a study showing that among 161 patients with PAF and normal left ventricular (LV) function, pulmonary vein isolation (PVI) resulted in stable sinus rhythm in 47% of patients after the first procedure, which increased to 80% when patients who had recurrent AF underwent additional ablation (Figure 1) [Ouyang F et al. Circulation. 2010]. Additionally, patients experienced a low progression to persistent forms of AF during 5 years of follow-up.
Clinical Outcomes After Each Ablation Procedure
Flowchart detailing the clinical outcome during 5-year follow-up after each ablation procedure.
Pts indicates patients; AF, atrial fibrillation; ATa, atrial tachyarrhythmia; FU, follow-up; SR, sinus rhythm.
Reprinted from Ouyang F et al, Long-term results of catheter ablation in paroxysmal atrial fibrillation: lessons from a 5-year follow-up, Circulation. 2010, Vol 122, Issue 23, Pages 2368-77A, with permission from American Heart Association, Inc.
According to Dr Wissner, one of the parameters that may drive the effectiveness of ablation is contact force (CF). CF between the catheter tip and the myocardium is important because too much force can cause perforation whereas too little CF creates a less durable lesion and gaps in the isolation line. Data from the EFFICAS I study [Neuzil P et al. Circ Arrhythm Electrophysiol. 2013]—in which the operators were blinded to CF readings—suggest that operators should aim for a target CF of 20 g, an absolute minimum CF of 10 g, and an absolute minimum Force-Time Integral (FTI) of 400 grams × seconds per individual ablation lesion in order to achieve successful isolation of the pulmonary veins.
The EFFICAS II study [Kautzner J et al. Europace. 2015] was a small multicenter study designed to prospectively assess whether CF guidance would reduce PVI gaps. Twenty-four patients in EFFICAS II were compared with 26 patients from EFFICAS I. The key result from EFFICAS II was that maintaining adequate CF levels (20 g, FTI ≥ 400 g × sec) and contiguous deployment of adjacent lesions resulted in a high proportion of durable PVI at 3-month follow-up. At follow-up, 85% of PVs remained isolated, compared with 72% in EFFICAS I (P = .04), in which the operators were blinded to CF readings.
Dr Wissner also discussed the second-generation cryoballoon, which is relatively easy to use, offers live verification of PVI, and can be used to create a one-shot transmural continuous lesion. In one study, 50 patients underwent PVI using the second-generation cryoballoon [Metzner A et al. Circ Arrhythm Electrophysiol. 2014]. Duration of the freeze cycle was 4 minutes plus 1 additional bonus freeze following successful isolation of the pulmonary vein. At a mean duration of 440 days, 80% of patients remained in normal sinus rhythm.
There is a paucity of data as to how to best use PVI in patients with persistent AF. In the STAR AF II study [Verma A et al. N Engl J Med. 2015], there was no significant difference in the rate of recurrent AF after 18 months of follow-up among patients who underwent either PVI alone, PVI plus linear ablation, or PVI plus ablation of complex fractionated electrograms (59% vs 46% vs 49%;P = .15). Hence, PVI alone may be the preferred strategy in patients with persistent AF. One-year clinical follow-up of 49 patients with persistent AF who underwent PVI with the second-generation cryoballoon and a bonus freeze showed that 69% remained in stable sinus rhythm [Lemes C et al. Europace. 2015].
In closing, Dr Wissner noted that PVI appears to be a viable option for patients with either paroxysmal or persistent AF. However, for patients who may need substrate modification, deployment of linear lesions, or have rotors, radiofrequency ablation is likely to be the better choice.
Another topic covered in this session is the use of cardiac resynchronization (CRT) for patients with depressed left ventricular ejection fraction. This therapy utilizes right atrial and ventricular pacing leads, in addition to a left ventricle lead advanced through the coronary sinus into a vein that runs along the ventricular free wall. This permits simultaneous pacing of both ventricles to allow resynchronization of the LV septum and free wall.
Angelo Auricchio, MD, PhD, Fondazione Cardiocentro Ticino, Lugano, Switzerland, reviewed the history of CRT. Prof Auricchio traced the roots of CRT to Dr M. Mower, who submitted a patent in 1989 for a “method and apparatus for treating hemodynamic function.” Although the patent was issued in May 1990, it took many more years for the concept to influence cardiology practice. According to Prof Auricchio, the feasibility of CRT has improved over the past 20 years, with most clinicians now able to accomplish 90% of CRT implantations in 90 minutes. This is due in part to the introduction of preshaped leads and more effective delivery catheters.
Another innovation in LV pacing was the invention of the open-ended and multipolar intravenous cardiac leads. Prof Auricchio then described the development of multifunctional leads, which allow electrophysiologists and cardiac surgeons more flexibility in programming and the ability to track wall motion directly from the implanted lead [Behar JM et al. J Cardiovasc Electrophysiol. 2015; Wecke L et al. Heart Rhythm. 2012].
Another breakthrough in the understanding of CRT is the use of endocardial pacing, which allows some patients to experience a full recovery of their LV structure and function. Although it is not clear why this mechanism is successful, results from canine models suggest that the benefit might be due to a shorter path length along the endocardium and faster impulse conduction during endocardial pacing of the left ventricle [Strik M et al. Circ Arrythm Electrophysiol. 2012]. Other animal studies have confirmed that endocardial pacing is superior to epicardial pacing [Bordacher P et al. Am J Physiol Heart Circ Physiol. 2012]. Prof Auricchio then focused on new wireless, ultrasound-based CRT systems, which were found to be a safe and viable option in 17 patients who had not previously undergone CRT implantation or who did not respond to CRT [Auricchio A et al. Europace. 2014]. In these patients, LV ejection fraction significantly increased (P < .01) at 6 months of follow-up.
Prof Auricchio closed his presentation by urging his colleagues to better understand how the electrical and mechanical differences impact the heart so they can consider strategies to deliver individualized resynchronization therapy.
- © 2015 SAGE Publications