Summary
Results of a phase 3 study indicate that adjunctive treatment with brivaracetam can significantly reduce the frequency of partial-onset seizures previously refractory to pharmacotherapy and improve responder rates. Patients receiving brivaracetam also had a higher rate of seizure freedom during treatment compared with those receiving placebo. Brivaracetam is safe and well tolerated.
- NCT01261325
- epilepsy
- partial-onset seizures
- brivaracetam
- adjunctive therapy
- episodic & paroxysmal disorders
- neurology clinical trials
Pavel Klein, MD, Mid-Atlantic Epilepsy and Sleep Center, Bethesda, Maryland, USA, presented the results of a phase 3 trial [NCT01261325] showing that adjunctive treatment with brivaracetam is associated with a significant reduction in the frequency of partial-onset seizures (POS) and improved response.
Brivaracetam is a selective high-affinity synaptic vesicle protein 2A ligand in clinical development for the treatment of epilepsy. The objective of this multicountry phase 3 study was to evaluate the efficacy, safety, and tolerability of brivaracetam compared with placebo as an adjunctive therapy in adult patients with focal epilepsy. Patients enrolled in this study had POS not fully controlled, despite treatment with 1 or 2 concomitant antiepileptic drugs. The study consisted of an 8-week baseline observational period and a 12-week treatment period, after which patients could undergo a 4-week down titration or enter into an open-label follow-up study. The co–primary efficacy outcomes were percentage reduction over placebo in 28-day adjusted seizure frequency and ≥ 50% responder rate based on percentage reduction in seizure frequency from baseline to the treatment period.
Patients aged ≥ 16 to 80 years with refractory POS who experienced ≥ 8 POS during the 8-week baseline period and ≥ 2 POS per month, with or without secondary generalization, during the 3 months prior to screening were recruited. Eligible patients were randomized 1:1:1 to placebo or brivaracetam 100 or 200 mg/d BID. During the 12 weeks of treatment, the rate of discontinuation was low and mostly due to adverse events (AEs; 3.8% in the placebo group and 8.3% and 6.8% among patients in the 100- and 200-mg/d brivaracetam groups, respectively). The majority of participants completed the study and entered the follow-up study. Patient disposition is shown in Table 1.
Patient Disposition
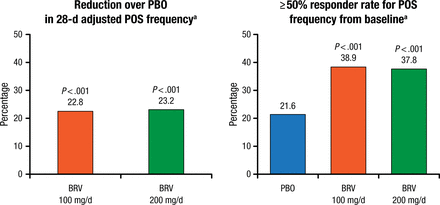
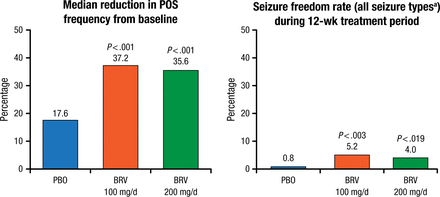
Both doses of brivaracetam significantly (P < .001) improved the co–primary outcomes of percentage reduction in POS and ≥ 50% responder rate adjusted to a 28-day duration compared with placebo (Figure 1). Patients receiving brivaracetam also had significantly higher median percentage reduction in seizures compared with baseline and significantly higher rates of seizure freedom (all types) during treatment (Figure 2).
Co–primary Efficacy Outcomes (Intention-to-Treat Population)
BRV, brivaracetam; PBO, placebo; POS, partial-onset seizures.
aDuring the treatment period.
Reproduced with permission from P Klein, MD.
Secondary Efficacy Outcome (Intention-to-Treat Population)
BRV, brivaracetam; PBO, placebo; POS, partial-onset seizures.
aPartial-onset, generalized, and unclassified seizures.
Reproduced with permission from P Klein, MD.
Treatment-emergent AEs occurred in 59.4% of placebo patients and 68.4% and 66.8% of patients in the 100- and 200-mg/d brivaracetam groups, respectively. The most common AEs (≥ 5%) were somnolence, followed by dizziness, fatigue, headache, and urinary tract infection; however, the latter 2 occurred with similar frequency in briviracetam- and placebo-treated patients. Serious treatment-emergent AEs occurred in < 3.5% of participants, without difference between the briviracetam and placebo treatment groups. There were 2 deaths, both in the brivaracetam 200-mg/d group; neither was considered related to the study drug.
This study confirms the results of 2 previous fixed-dose phase 3 trials that used 5- to 100-mg/d doses of brivaracetam [Biton V et al. Epilepsia. 2014; Ryvlin P et al. Epilepsia. 2014].
- © 2015 SAGE Publications