Summary
Stroke is a serious condition that can result in substantial morbidity and mortality. It is the number one cause of disability and the second-most common cause of death in the world [World Health Organization.The Atlas of Heart Disease and Stroke 2004]. A major underlying cause of stroke in many patients is atrial fibrillation (AF). This article discusses the role of AF in cryptogenic stroke.
- Cerebrovascular Disease
- Arrhythmias
- Cardiology & Cardiovascular Medicine
- Cerebrovascular Disease
- Arrhythmias
Stroke is a serious condition that can result in substantial morbidity and mortality. It is the number one cause of disability and the second-most common cause of death in the world [World Health Organization. The Atlas of Heart Disease and Stroke 2004]. A major underlying cause of stroke in many patients is atrial fibrillation (AF). Hakop Hrachian-Haftevani, MD, Baptist Health International, Miami, Florida, USA, discussed the role of AF in cryptogenic stroke.
There are multiple potential causes of cardioembolic stroke, including AF, low ejection fraction, and cardiac thrombus, as well as rarer events, such as cardiac tumor, vegetation on cardiac valves, mobile aortic arch atheroma, endocarditis, and patent foramen ovale with an embolism from a venous source. However, if the workup is negative for the preceding etiologies and there is no history of AF, then the stroke is deemed cryptogenic, and the patient is considered to be at a high risk of stroke recurrence, disability, and death.
AF increases the risk of stroke 5-fold, with similar risk among patients with paroxysmal or permanent AF [Hart RG et al. J Am Coll Cardiol 2000]. However, up to 90% of paroxysmal AF is asymptomatic, and even a few minutes a month of AF episodes is sufficient to cause stroke [Israel CW et al. J Am Coll Cardiol 2004]. In addition, 25% of AF-related strokes occur in patients with no history of AF. Therefore, identifying AF is important and can change the treatment of cryptogenic stroke—namely, by treating patients with oral anticoagulants (OACs) instead of antiplatelet agents [Camm AJ et al. Eur Heart J 2012].
In determining whether AF is present, it is important to not rely on patients' symptoms, because there is a poor association between their perceptions of symptoms and actual AF events [Strickberger SA et al. Heart Rhythm 2005]. However, short-term or intermittent monitoring may not be adequate to record AF-related events. In a study showing a subgroup analysis of the TRENDS trial [Glotzer TV et al. Circ Arrhythm Electrophysiol 2009], 60% of patients with stroke had newly detected AF identified ≥ 30 days after the stroke, which was recorded by a continuous monitoring via a pacemaker or implantable cardioverter defibrillator [Ziegler PD et al. Stroke 2010]. Importantly, there is a 4-fold higher risk of stroke recurrence in patients with newly detected AF compared with patients with known AF or no AF [Kamel H et al. J Stroke Cerebrovasc Dis 2009].
In a retrospective analysis of 574 patients with pacemakers who were known to have AF, monitoring that was performed on randomly selected days to simulate intermittent monitoring proved to be inaccurate in identifying AF [Ziegler PD et al. Heart Rhythm 2006]. In contrast, the SURPRISE study [NCT01498146] monitored 84 patients with cryptogenic stroke or transient ischemic attack (TIA) with no known AF, with an implanted Reveal XT for up to 3 years [Christensen LM et al. ISC 2013 (abstr 209)]. The results indicated AF in 15.5% of the patients, with a median time of 106 days from stroke onset to the first recorded AF-related event.
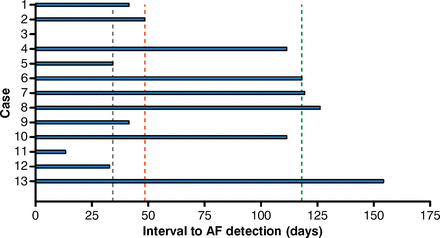
In a study that used an implantable loop recorder, AF was identified in 25.5% of patients with cryptogenic stroke [Cotter PE et al. Neurology 2013]. The median time to AF detection was 48 days following the implant, with the first detected episode lasting a median of 6 minutes (Figure 1).
Detection of Atrial Fibrillation With an Implantable Loop Recordera
a Dashed lines represent median and interquartile ranges. Case 3 had a delay of 0 days (AF on postimplantation device check).
AF=atrial fibrillation.
Reproduced from Cotter PE et al. Incidence of atrial fibrillation detected by implantable loop recorders in unexplained stroke. Neurology. 2013;80(17):1546–1550. With permission from Lippincott Williams and Wilkins/Wolters Kluwer Health.
Another study demonstrated that an implantable cardiac monitor (ICM) identified AF in 17% of patients with cryptogenic stroke compared with 1.7% identified by a 7-day Holter monitor (p = .0077) [Ritter MA et al. Stroke 2013]. The mean time to detection of AF was 64 days following ICM implantation.
The prospective randomized CRYSTAL-AF study [Sanna T et al. N Engl J Med 2014] evaluated ICM versus the standard of care in the detection of AF in patients with cryptogenic stroke with no known AF. The primary end point of AF at 6 months was detected in 8.9% of the ICM arm and 1.4% of the control arm (HR, 6.4; 95% CI, 1.9 to 21.7; p < .001. At 36 months, AF was detected in 30% and 3% of the ICM and control arms, respectively (HR, 8.8; 95% CI, 3.5 to 22.2; log-rank p < .0001). These data suggest that long-term continuous monitoring is needed to better identify AF in patients with cryptogenic stroke.
One type of ICM is the Reveal XT, which can be implanted in 20 minutes under local anesthesia and explanted in 10 minutes, if required. There are no wires or leads; it is compatible with magnetic resonance imaging; and it does not restrict activity or movement. Reveal XT can be accessed by CareLink, and it is able to detect AF at heart rates > 100 beats per minute. Similarly, the Reveal LINQ is substantially smaller and thinner than the Reveal XT and is implanted with a special needle-like tool. Both devices have been approved by the US Food and Drug Administration for use in patients with clinical syndromes or situations that increase their risk of cardiac arrhythmias and for patients who experience symptoms that suggest cardiac arrhythmia.
Patients with AF who experience stroke have an higher risk of morbidity and mortality compared with patients without AF who experience stroke, likely due to the severity of the event or comorbidities [Dulli DA et al. Neuroepidemiology 2003]. Therefore, early identification of AF is important, as well as the prevention of stroke in this population. Theodore Wein, MD, Montreal General Hospital, Montreal, Canada, discussed the effects of AF-related stroke and the role of OACs.
Warfarin is an OAC that has been in clinical use for > 60 years, and it reduces the risk of AF-associated stroke by about two-thirds [Hart RG et al. Ann Intern Med 2007; Connolly S et al. Lancet 2006]; however, warfarin also increases the risk of hemorrhage, including intracranial hemorrhage [Gomes T et al. CMAJ 2013].
Despite the data favoring warfarin use to prevent stroke in patients with AF, one study found that only 10% of high-risk patients with AF and 18% of high-risk patients with AF and a history of stroke or TIA received therapeutic warfarin [Gladstone DJ et al. Stroke 2009]. More recently, dabigatran, rivaroxaban, and apixaban were approved for the prevention of stroke in patients with nonvalvular AF, all of which were at least equivalent to warfarin in terms of stroke prevention and bleeding risk [Granger CB et al. N Engl J Med 2011; Patel MR et al. N Engl J Med 2011; Connolly SJ et al. N Engl J Med 2010]. Prof. Wein stated that, in his opinion, based on indirect comparison, dabigatran (150 mg, twice per day [bid]) is best for stroke prophylaxis, followed by apixaban (5 mg, bid), dabigatran (110 mg, bid), then rivaroxaban (20 mg), and that the agent with the lowest risk of bleeding is apixaban, followed by dabigatran (110 mg, bid), dabigatran (150 mg, bid), then rivaroxaban (20 mg).
Prof. Wein concluded by pointing out that a major reason why the novel OACs provide a greater benefit than warfarin for many patients is due to the reduced risk of intracerebral hemorrhage, which in turn means that patient populations who may not have been considered for OAC may now benefit from therapy with a novel OAC.
In conclusion, cryptogenic stroke is frequently a result of undetected AF, and prolonged or intensive monitoring may be necessary to detect AF. It is important that AF be detected early to prevent stroke recurrence, which is best done with a long-term continuous monitor such as an ICM. If AF is detected, many patients will benefit from stroke prophylaxis with an OAC. The novel OACs should be considered over warfarin because of the reduced risk of intracerebral hemorrhage and at least equivalent risk of stroke, if not lower.
- © 2014 MD Conference Express®