Summary
This article presents the relevant radiographic data for the Efficacy at 24 Weeks and Long-Term Safety, Tolerability, and Efficacy up to 2 Years of Secukinumab (AIN457) in Patients With Active Psoriatic Arthritis (PsA) trial [FUTURE 1; NCT01392326]. Analysis specifically focuses on one of the secondary outcomes of the study, radiographic analysis at week 24 (primary analysis) and radiographic analysis at week 52 (secondary analysis).
- Radiography
- Rheumatology Clinical Trials
- Arthritis
- Inflammatory Disorders
- Radiography
- Rheumatology Clinical Trials
- Arthritis
- Inflammatory Disorders
- Rheumatology
In patients with psoriatic arthritis (PsA) treated with secukinumab, radiographic progression of joint structural damage at 24 weeks was significantly inhibited compared with placebo, an effect sustained through 52 weeks of treatment. Desiree van der Heijde, MD, Leiden University Medical Center, The Netherlands, presented the relevant radiographic data for the Efficacy at 24 Weeks and Long-Term Safety, Tolerability, and Efficacy up to 2 Years of Secukinumab (AIN457) in Patients With Active Psoriatic Arthritis (PsA) trial [FUTURE 1; NCT01392326]. Prof van der Heijde focused on one of the secondary outcomes of the study, radiographic analysis at week 24 (primary analysis) and radiographic analysis at week 52 (secondary analysis).
FUTURE 1 was a multicenter, randomized, placebo-controlled phase 3 study in which 606 patients with PsA received an intravenous loading dose of secukinumab at 10 mg/kg every 2 weeks for the first 4 weeks, followed by subcutaneous doses of 75 mg (n = 202) or 150 mg (n = 202) monthly, compared with placebo (n = 202).
Radiographic assessment was based on the modified total Sharp score (mTSS), with total scores ranging from 0 to 528, consisting of erosion scores (maximum score of 360) and joint space narrowing (JSN) scores (maximum score of 168); the higher scores indicate more articular damage. Baseline characteristics of the 3 treatment groups (secukinumab 150 mg, secukinumab 75 mg, and placebo), focusing on the radiographic variables, showed that the mean mTSS was about 20 in all groups, albeit a little higher in the placebo group; roughly half of this score was due to erosions and half to JSN. About 70% of patients were anti-tumor necrosis factor (TNF) naïve, and about 60% were using concurrent methotrexate (MTX).
Using the mTSS, radiographic assessment was done on each hand/wrist and foot at baseline and at weeks 16/24 and 52. Among radiographic completers, 75% of patients completed radiographs up to 52 weeks and > 94% completed radiographs up to 24 weeks.
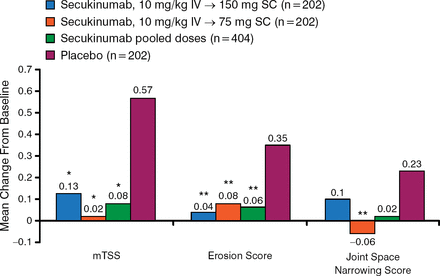
The results of the study found that secukinumab significantly inhibited radiographic disease progression at week 24 compared with placebo, both when looking at the combined mTSS of the 150 and 75 mg secukinumab groups as well as each dose group separately (all P < .05; Figure 1).
Secukinumab Significantly Inhibits Radiographic Disease Progression at Week 24
IV, intravenous; mTSS, modified total Sharp score; SC, subcutaneous.
*P < .05 vs placebo (P values at week 24 adjusted for multiplicity of testing).
**P < .05 vs placebo. Reproduced with permission from D van der Heijde, MD.
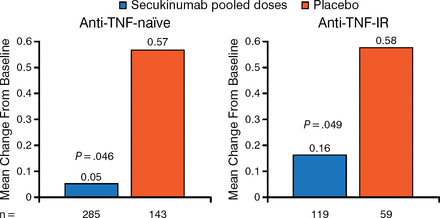
When looking at a prespecified analysis of radiographic progression based on anti-TNF status, the study found significant radiographic progression inhibition in patients treated with secukinumab regardless of TNF status (Figure 2).
Significant Radiographic Progression Inhibition With Secukinumab Regardless of Tumor Necrosis Factor Status
Full analysis set using nonparametric analysis of covariance with linear extrapolation for missing values at week 24; anti-TNF-IR: subjects with inadequate response or intolerance to previous therapy with an anti-TNF.
IR, inadequate responder; TNF, tumor necrosis factor.
Reproduced with permission from D van der Heijde, MD.
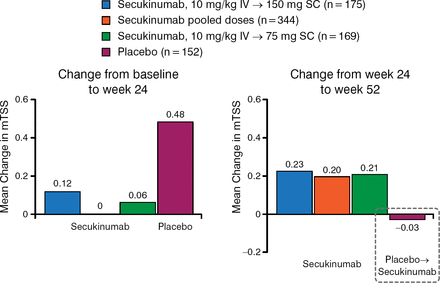
The study also found that patients treated with secukinumab had sustained inhibition of radiographic disease progression from baseline through week 52, and that radiographic disease progression was inhibited between 24 and 52 weeks in patients initially treated with placebo who switched to secukinumab (Figure 3).
Inhibition of Radiographic Disease Progression Sustained Through Week 52 With Secukinumaba
IV, intravenous; mTSS, modified total Sharp score; SC, subcutaneous.
Radiographic disease progression was inhibited in placebo-treated subjects upon switching to secukinumab.
aX-ray completers (those subjects who had X-ray measures at baseline, week 16/24, and week 52). Reproduced with permission from D van der Heijde, MD.
The inhibition of structural damage seen with secukinumab was seen in patients regardless of prior treatment with TNF inhibitor, or concurrent MTX use.
- © 2014 MD Conference Express®