Summary
Considerable progress has been made in multiple sclerosis (MS) therapy in the 20 years since the first successful trial. Although several agents are now available for treating patients with MS, many issues remain regarding treatment of individual patients. This article discusses initiating MS therapy, and addressed the issues of switching and escalating therapy and of discontinuing therapy.
- Demyelinating Diseases
- Exclusive Article - For home page
- Demyelinating Diseases
- Neurology
- Exclusive Article - For home page
Considerable progress has been made in multiple sclerosis (MS) therapy in the 20 years since the first successful trial. Although several agents are now available for treating patients with MS, many issues remain regarding treatment of individual patients. Fred D. Lublin, MD, Icahn School of Medicine at Mount Sinai, New York, New York, USA, opened the session with his presentation on initiating therapy. B. Mark Keegan, MD, and Brian G. Weinshenker, MD, both of the Mayo Clinic, Rochester, Minnesota, USA, addressed the issues of switching and escalating therapy and of discontinuing therapy, respectively.
INITIAL CHOICE OF THERAPY
Since the first MS therapy became available 20 years ago, treatment of MS has been initiated earlier and earlier. Dr. Lublin addressed the difficult questions of who to treat, when to treat, and what drug should be used to initiate therapy.
The greatest strides have been made in treating patients with clinically active relapsing MS. All of the current US Food and Drug Administration (FDA)-approved disease-modifying therapies (DMT) have been tested in clinical trials of patients with relapsing MS. These studies have demonstrated a benefit in reducing relapses and magnetic resonance imaging (MRI) activity, and in some cases, reducing accumulation of disability. Earlier treatment results in better outcomes. Initial treatment of patients with secondary progressive (SP) or primary progressive (PP) MS is more problematic, as little evidence for successful therapy exists unless activity is present. [Tullman MJ. Am J Manag Care 2013; Miller AE et al. Curr Opin Neurol 2012].
Treatment for patients with clinically isolated syndrome (CIS)—an acute single episode—is a challenge if the MRI is normal [Miller DH et al. Lancet Neurol 2012]. Such patients have only a 20% chance of another clinical event over the next 2 decades if their brain MRI is normal, but patients with ≥1 MRI lesions have an 80% chance. Thus, if the MRI is abnormal, the evidence shows that initiating treatment will reduce the risk of additional attacks. Patients with radiologically isolated syndrome (RIS) present the greatest challenge. These patients may experience subsequent clinical or radiologic events, but little evidence exists regarding treatment in this population [Okuda DT et al. Neurology 2009].
Disease modifying therapy (DMT) agents with 7 different anti-inflammatory mechanisms are approved for relapsing MS in the United States (see Table 1) [Tullman MI. Am J Manag Care 2013; Miller AE et al. Curr Opin Neurol 2012]. All have good clinical trial data to support their use. Head-to-head comparative studies provide the best evidence for assessing efficacy, but few have been done.
Food and Drug Administration Approved DMTs for Multiple Sclerosis
Factors considered in choosing an initial therapy include comparative trial data, mechanism of action, efficacy, safety, disease characteristics, biomarkers, prior therapies, comorbidities, and patient convenience. Further studies are needed to obtain long-term and good comparative efficacy data, as well as data on defining inadequate response and switching therapies.
SWITCHING AND ESCALATING THERAPY
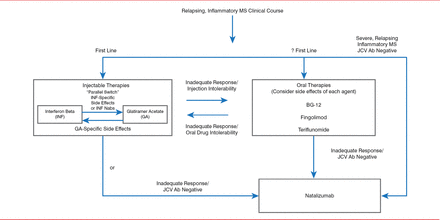
According to Dr. Keegan, the optimal therapeutic management strategy for MS, including switching and escalating therapy, relies on an accurate diagnosis of MS and identifying the clinical course. Therapeutic goals include reducing clinical relapses and MRI inflammatory lesions, reducing short-and long-term disability, achieving a tolerable side effect profile, and meeting safety-monitoring requirements. Patients should be assessed to determine if these goals have been achieved and if therapy should be switched or escalated (Figure 1) [Keegan BM. Semin Neurol 2013].
Algorithm for Assessing Multiple Sclerosis Therapy
Ab=antibody; BG-12=dimethyl fumarate; JCV=!ohn Cunningham virus; Nab=neutralizing antibody.
Reproduced from Keegan BM et al. Therapeutic decision making in a new drug era in multiple sclerosis. Semin Neurol 2013;33(1):5–12. With permission from Thieme Medical Publishers.
Switching medications within a drug class or out of a class across the same level of therapy is called parallel switching. An example of parallel switching is switching between interferon beta (IFNb) and glatiramer acetate (GA) because of IFNb- or GA-specific side effects or IFNb neutralizing antibodies. A route switch between an oral and an injectable therapy may be made in cases of inadequate response or intolerability to an oral or injectable drug. Efficacy and side effects of the drugs should be considered when making such a switch.
For patients with an inadequate response to injectable or oral therapies who are John Cunningham virus (JCV) antibody negative, therapy can be escalated by switching to natalizumab, which has a strong anti-inflammatory effect. JCV causes the opportunistic infection, progressive multifocal leukoencephalopathy (PML), which results in severe disability or death [Tan et al. Neurology 2011]. Approximately 54% of MS patients test positive for JCV, and the annual seroconversion rate is about 2% [Berger JR et al. Ann Neurol 2013; Gorelik L et al. Ann Neurol 2010]. “De-escalating” therapy from natalizumab to an oral medication can be done after a washout period of <3 months [Cohen M et al. JAMA Neurol 2014], with a low relapse risk [Jokubaitis VG et al. Neurology 2014].
DISCONTINUING THERAPY
Patients with MS may discontinue therapy for a variety of reasons. Based on data from studies of MS DMT discontinuation, Dr. Weinshenker concluded that early discontinuation, typically within 5 years of initiation of IFNb and GA is common (Table 2). Although patients often switch to other treatments, approximately 20% permanently discontinue treatment. Lack of efficacy is the most commonly cited factor. Tolerability issues are common reasons for stopping, but serious safety reasons are relatively uncommon.
Studies Examining Rates of Discontinuing Multiple Sclerosis DMT
Dr. Weinshenker categorized reasons for discontinuing as good, reasonable, or bad. Good reasons include genuine lack of efficacy, serious toxicity, and pregnancy. Reasonable reasons include high titer of IFNb neutralizing antibodies, poor tolerance, a long period of no evidence of disease activity in patients >50 years of age, and entry into the progressive MS phase. Bad reasons include misperceptions about treatment goals, nihilistic approach to treatment, assumption that treatment is curative rather than partially effective, inadequate education about adverse effect management and duration, and cost or insurance issues.
Early discontinuation of therapy is common but the rate of late discontinuation is not well studied. Therapy is unsuccessful in a large proportion of patients in clinical trials, and the main reason cited for early discontinuation is lack of efficacy. Prospective studies of discontinuation of DMTs integrated with algorithms of treatment escalation based on evidence of ongoing inflammatory disease activity are needed to guide decisions on stopping or switching therapy.
Whether treatment should be stopped when MS becomes progressive is unknown, but might be inferred because all DMT's are approved for patients with relapsing forms of MS. DMTs that have been evaluated in patients with progressive MS have been shown to have limited efficacy except in those with superimposed relapses or MRI evidence of disease activity [Kappos L et al. Neurology 2004].
Success rates for stopping DMT in stable patients and predictors of success have also not been studied. In the absence of evidence, Dr. Weinshenker requires a 7-year period of freedom from disease activity before approving a patient's decision to discontinue treatment, while advising the patient that the safety of discontinuation is unknown.
- © 2014 MD Conference Express®