Summary
The 2014 American Heart Association/American College of Cardiology guidelines for the management of patients with valvular heart disease include new definitions of disease severity and provide for a more in-depth assessment of risk and guidance on the choice of optimal therapy, particularly with respect to transcatheter aortic valve replacement. The format of the guidelines has also been improved to improve their use in clinical practice.
- TAVR
- SAVR
- mitral regurgitation
- aortic stenosis
- heart team
- guidelines
- cardiology & cardiovascular medicine guidelines
- valvular disease
- interventional techniques & devices
The 2014 American Heart Association/American College of Cardiology (ACC) guidelines for the management of patients with valvular heart disease [Nishimura RA et al. J Thorac Cardiovasc Surg. 2014; Circulation. 2014; J Am Coll Cardiol. 2014] are based on new data on the natural history of the disease, improvements in imaging that allow for better quantitation of stenosis and valve regurgitation, and better outcomes from surgical and catheter-based interventions. This new information allows for a lower threshold for intervention and extends treatments to sicker populations. Rick A. Nishimura, MD, Mayo Clinic College of Medicine, Rochester, Minnesota, USA, discussed key aspects of these guidelines.
Stages of Disease
Similar to the guidelines for heart failure, the 2014 valvular disease guidelines now consider disease stage. The authors identified 4 stages of increasing disease severity: stage A identifies individuals at risk for disease; stage B defines those with progressive disease; stage C includes individuals with severe but asymptomatic disease; and stage D is severe symptomatic disease. Severe disease is defined as the presence of symptoms or when natural history studies show a poor outcome.
According to the guidelines, observation and monitoring are appropriate for patients with stage A or B disease. Stage C patients should be further risk stratified through an assessment of left ventricular (LV) function. High-risk stage C and stage D patients warrant intervention.
With aortic stenosis (AS) as an example, peak aortic jet velocity (AV-Vel) is a predictor of outcome in patients with AS and can be used to evaluate disease severity. Even among patients with asymptomatic AS, an AV-Vel > 4.0 m/s is considered severe, while a velocity > 5.0 m/s is considered very severe [Rosenhek R et al. Circulation. 2010]. Stage C patients with decompensated LV function (end-diastolic pressure ≥ 40 mm) have worse outcomes. Intervention is recommended for patients with stage C disease and decompensated LV function and for those with stage D disease (severe symptomatic AS). The appropriate treatment for patients with stage C disease and compensated LV function is unclear.
Guidelines in the 21st Century
Practicing physicians need concise relevant bytes of knowledge synthesized by an expert that answer specific clinically relevant questions. Although the guidelines have the information, the format has not supported this need in the past. The 2014 valve disease guidelines were written with the needs of the practicing physician in mind. The taxonomy and evidence tables are based on how clinicians think (ie, diagnosis and testing, medical therapy, and treatment intervention). The ultimate objective is for the guidelines to contain supporting text with links to references and figures. Dr Nishimura believes that guidelines should be viewed as a living document for the 21st century where new knowledge can be added to the guidelines in a continuous stream.
Lower Threshold for Intervention
Catherine M. Otto, MD, University of Washington, Seattle, Washington, USA, reviewed how the guidelines assess asymptomatic disease and low-flow AS. Although there is general agreement that it is appropriate to intervene once patients with AS become symptomatic (eg, angina, syncope, heart failure), prior to symptom onset, the clinician must balance the risk of monitoring and waiting with risk of aortic valve replacement (AVR).
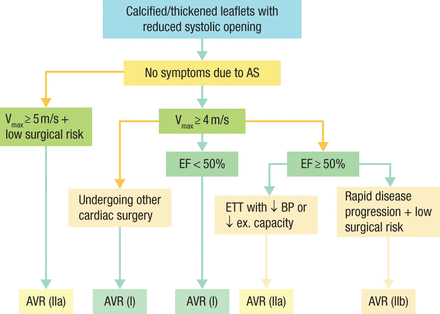
For most patients with severe asymptomatic AS, monitoring is an acceptable course, but it is important to establish whether a patient is truly asymptomatic, whether his or her LV systolic function is normal, whether the AS is very severe or rapidly progressive, and what risks exist for intervention for this patient. An evaluation for symptoms includes a careful history and the identification of early diagnostic symptoms, such as decreased exercise tolerance, dyspnea, and exertional dizziness. If there is any uncertainty, stress testing is recommended. LV systolic function should be evaluated to determine the level of compensation, and it is important to remember that older patients are more likely to progress rapidly. Moderate or severe valvular calcification and an AV-Vel > 5.0 m/s identify patients with a very poor prognosis [Rosenhek R et al. N Engl J Med. 2010; Circulation. 2010]. Patients with asymptomatic severe AS, severe calcification, rapid progression, and LV ejection fraction (EF) < 50% will benefit from early AVR (Figure 1).
2014 Timing of Intervention in Asymptomatic Patients With Severe AS
AS, aortic stenosis; AVR, aortic valve replacement; BP, blood pressure; EF, ejection fraction; ETT, exercise tolerance test; ex, exercise; Vmax, velocity.
Reproduced with permission from CM Otto, MD.
Source: Nishimura RA et al. J Thorac Cardiovasc Surg. 2014.
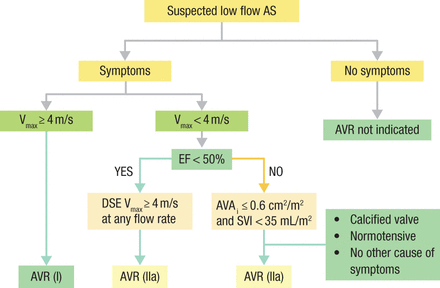
The guidelines also outline the timing and indications for intervention for the different hemodynamics and symptoms, such as low-flow AS (Figure 2).
ACC/AHA Valve Guidelines for Low-Flow AS
ACC, American College of Cardiology; AHA, American Heart Association; AS, aortic stenosis; AVAi, aortic valve area index; AVR, aortic valve replacement; DSE, dobutamine stress echocardiogram; EF, ejection fraction; SVI, stroke volume index; Vmax, velocity.
Reproduced with permission from CM Otto, MD.
Source: Nishimura RA et al. J Thorac Cardiovasc Surg. 2014.
In all cases, patient preferences and values should also be considered. As heart valves improve and procedural risks decrease, interventions are likely to be applied earlier in the course of disease.
Primary vs Secondary MR
Blase A. Carabello, MD, Mount Sinai Beth Israel, New York, New York, USA, reminded the audience of the importance of understanding the difference between primary (organic) and secondary (functional) mitral regurgitation (MR). For primary MR, the triggers for intervention are EF < 60%, a pulmonary artery pressure of 50 mm Hg, or an end-systolic dimension of ≥ 40 mm. The emphasis should be on early durable repair. The development of even mild symptoms by the time of surgical referral is associated with reduced survival outcomes in patients with severe primary MR [David TE et al. Circulation. 2013; Gillinov AM et al. Ann Thorac Surg. 2010]. The best time to operate on these patients is before symptoms develop (provided valve pathology indicates that a repair is almost certain) and before LV end-systolic diameter reaches 40 mm, noted Dr Carabello [Tribouilloy C et al. J Am Coll Cardiol. 2009], or before EF declines to 60%.
The worse the pulmonary hypertension, the worse the short- and long-term survival is after MR surgery. Ghoreishi et al [J Thorac Cardiovasc Surg. 2011] found that in patients undergoing MR surgery, operative mortality was 2%, 3%, 8%, and 12% for those with no, mild, moderate, and severe preoperative pulmonary hypertension, respectively.
Durable repair is a key for long-term survival. Most surgeons would consider surgery for a patient with no symptoms, normal LV function (EF > 60%), and an end-systolic dimension < 40 mm when there is a 95% likelihood of successful repair (class IIa).
Secondary MR is virtually a separate disease from primary MR. Because it is secondary to severe LV dysfunction, it is associated with a poor prognosis; therefore, it is not surprising that no studies have shown improvement in survival following mitral valve surgery (MVS) compared with medical therapy or when MVS was added to bypass surgery [Benedetto U et al. J Cardiovasc Med. 2009]. For some patients with secondary MR, aggressive medical therapy, including cardiac resynchronization therapy, can be helpful [van Bommel RJ et al. Circulation. 2011]. Although there is lack of survival benefit, patients do feel better after surgery as demonstrated by an improvement in NYHA class. Unlike for primary MR, in patients with secondary MR, surgery should be performed after all else has been tried.
The Heart Team
Many practice guidelines—including those of the European Society of Cardiology, the European Association for Cardio-Thoracic Surgery, the Centers for Medicare and Medicaid Services, and the ACC—recommend the use of a multidisciplinary heart team consisting of a clinical/noninvasive cardiologist, an interventional cardiologist, and a cardiac surgeon (class I, level C). Michael Mack, MD, Baylor Scott & White Health, Dallas, Texas, USA, supports this position and discussed how the use of such a team can help to ensure the selection of an optimal treatment strategy.
Currently there is a lack of consensus regarding the use of the heart team approach in terms of its definition, composition, desired goals, means of implementation, metrics of success, and unintended consequences [Coylewright M et al. J Am Coll Cardiol. 2015. In press]. Dr Mack described 5 reasons to have a heart team and 4 reasons not to (Table 1).
Support for and Against Utilizing a Heart Team
The makeup of a heart team can vary. The patient, cardiologist, surgeon, imagers, anesthesiologist, midlevel providers, and lead coordinator compose a typical team. In most practices, the team will evolve as needed, perhaps adding a neurologist or electrophysiologist.
The heart team should meet on a regular basis but also as needed. It should be organized into integrated practice units with the following characteristics:
Clinical and nonclinical personnel providing full-cycle care for a condition
Dedicated multidisciplinary team
Outpatient, inpatient, rehabilitative care integrated
Single administrative and scheduling unit
Joint accountability for outcomes and costs
Team members should be in continuous contact via e-mail and text messaging. A fully integrated heart team offers the best patient outcomes at the best cost.
Timing of Intervention With TAVR
As outcomes have improved, transcatheter aortic valve replacement (TAVR) has moved from being used only for the sickest patients toward being the first choice for AS therapy in most patients. Vinod H. Thourani, MD, Emory University, Atlanta, Georgia, USA, discussed the indications and timing of TAVR intervention.
TAVR with medical therapy is superior to medical therapy alone in inoperable patients, equivalent to surgical aortic valve replacement (SAVR) in high-risk patients, and may be equivalent to SAVR in intermediate- and low-risk patients. It is generally agreed that a less invasive therapy is preferable to a more invasive approach when the 2 have equivalent outcomes; however, it is still necessary to assess whether the trade-off is worth the less invasive approach.
Outcomes after SAVR are improving. One recent study in almost 142 000 patients showed significantly improved in-hospital mortality (P < .0001) in 80% of patients compared with the predicted risk [Thourani VH et al. Ann Thorac Surg. 2015]. When a treatment course for AVR is being chosen, the level of organ dysfunction is also an important consideration, as multiple organ dysfunctions significantly decrease short- and long-term survival [Thourani VH et al. Ann Thorac Surg. 2013].
In a head-to-head comparison of SAVR and TAVR in the PARTNER I trial, there were no differences in all-cause mortality, median survival, or mean gradient [Mack M et al. ACC 2015]. Recent reports from the PARTNER II trial, however, show significant improvement in 30-day mortality with the Sapien 3 valve [Kodali S et al. ACC 2015].
Dr Thourani sees TAVR becoming more common among intermediate-risk patients, with most low-risk patients receiving SAVR. More data are needed on the appropriate therapy for frail patients and for futility.
- © 2015 SAGE Publications