Summary
Resistance of multidrug resistant Gram-negative pathogens to carbapenems is increasing. The value of ceftazidime and avibactam as an alternative to carbapenems was explored in the REPRISE trial. The results indicate that the combination of ceftazidime and avibactam is tolerable and effective, with clinical success similar best available therapy (typically carbapenem monotherapy).
- REPRISE
- NCT01644643
- ceftazidime
- avibactam
- urinary tract infection
- carbapenem
- complicated intra-abdominal infection
- bacterial infections
- infectious diseases clinical trials
Urinary tract infections (UTIs) due to multidrug-resistant gram-negative bacteria (including ceftazidime resistant) respond to treatment via a combination of ceftazidime and avibactam. The results of REPRISE [NCT01644643], a prospective open-label phase 3 trial, were presented by Yehuda Carmeli, MD, MPH, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel.
The burgeoning prevalence of multidrug-resistant gram-negative pathogens spurred the use of carbapenems, but resistance to carbapenems is also spreading. Ceftazidime-avibactam (CAZ-AVI) may have merit as an alternative, and it was the focus of the REPRISE trial.
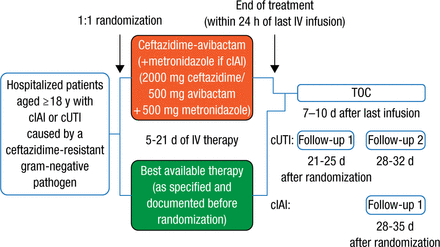
Patients aged ≥ 18 years who were hospitalized with complicated intra-abdominal infection (cIAI) or complicated urinary tract infection (cUTI) due to ceftazidime-resistant gram-negative bacteria were randomized 1:1 for 5 to 21 days of intravenous therapy involving either best available therapy (BAT; carbapenem antibiotic monotherapy in 97% of cases) or CAZ-AVI (followed by metronidazole in the case of cIAI that could involve anaerobes), with dose reduction for patients with renal impairment. Treatment outcome (test of cure [TOC]) was ascertained 7 to 10 days after the last treatment in the microbiologically modified intention-to-treat (mMITT) population (Figure 1).
Study Design
cIAI, complicated intra-abdominal infection; cUTI, complicated urinary tract infection; IV, intravenous; TOC, test of cure.
Reproduced with permission from Y Carmeli, MD, MPH.
Ceftazidime-resistant Enterobacteriaceae and Pseudomonas aeruginosa were defined as having a ceftazidime minimal inhibitory concentration of ≥ 8 and ≥ 16 mg/L, respectively. The primary end point was the clinical response to treatment. Secondary end points included favorable microbiological response in the mMITT population and safety, as determined by emergent adverse events (AEs) and laboratory testing.
The 53-center, 16-country trial involved 333 patients randomized to CAZ-AVI (n = 165; cUTI, n = 153) or BAT (n = 168; cUTI, n = 153). The mMITT population comprised 302 patients (CAZ-AVI, n = 154; BAT, n = 148). At baseline, the characteristics were generally similar in the cUTI patients in both groups. Patients with cIAI were broadly similar, considering the small number of patients (Table 1).
Baseline Characteristics of the Study Groups
The majority of patients were infected with Enterobacteriaceae, most commonly Escherichia coli and Klebsiella pneumoniae. The overall clinical cure rate (cUTI and cIAI) at TOC in the mMITT population was 140 of 154 (90.9%; 95% CI, 85.6% to 94.7%) for CAZ-AVI and 135 of 148 (91.2%; 95% CI, 85.9% to 95.0%) for BAT. For patients with cUTI, the clinical cure rate at TOC in the mMITT population was 132 of 144 (91.7%; 95% CI, 86.3% to 95.4%) for CAZ-AVI and 129 of 137 (94.2%; 95% CI, 89.3% to 97.2%) for BAT. The per-patient favorable microbiological response rate in patients with cUTI treated with CAZ-AVI (n = 118 of 144, 81.9%; 95% CI, 75.1% to 87.6%) was higher than that with BAT (n = 88 of 137, 64.2%; 95% CI, 56.0% to 71.9%). Rates of clinical cure declined with time but remained ≥ 85% for the CAZ-AVI arm.
AEs occurred in 51 of 164 (31.1%) and 66 of 168 (39.3%) patients in the CAZ-AVI and BAT arms, respectively, with serious AEs in 5.5% and 6.0% of patients, respectively. The most frequent AEs were gastrointestinal disorders (12.8% and 17.9%, respectively). Seven deaths (3 in the CAZ-AVI arm and 4 in the BAT arm) were not considered related to the therapy.
The results indicate the potential value of CAZ-AVI in the treatment of cUTI caused by ceftazidime-resistant gram-negative bacteria. The small numbers of cIAI patients preclude any definitive conclusion about the efficacy of CAZ-AVI in treating this sort of infection.
- © 2015 SAGE Publications