Summary
In the open-label, phase 3 RE-VERSE AD trial, idarucizumab resulted in complete reversal of dabigatran-related anticoagulation in up to 88% of patients with uncontrolled bleeding or who required an emergent procedure, with no safety concerns. The median maximum reversal of 100% occurred within 4 hours and was evident within minutes after the first of 2 idarucizumab infusions.
- RE-VERSE AD
- NCT02104947
- dabigatran
- idarucizumab
- anticoagulation
- cardiology & cardiovascular medicine clinical trials
- thrombotic disorders
Idarucizumab, an antidote for dabigatran, achieved immediate and complete reversal of dabigatran-associated anticoagulation among patients with uncontrolled bleeding or those requiring an emergent procedure. There were no associated safety concerns. Charles V. Pollack, MD, Thomas Jefferson University, Philadelphia, Pennsylvania, USA, presented the initial results from the RE-VERSE AD trial [Pollack CV et al. N Engl J Med. 2015].
Although some dosing regimens of dabigatran have been associated with fewer serious bleeding events compared with warfarin [Eikelboom JW et al. Circulation. 2011], life-threatening anticoagulation-associated bleeding events can still occur. In addition, some patients on dabigatran may require emergent procedures [Graham DJ et al. Circulation. 2015]. Currently, there are no approved specific reversal strategies for the management of these patients. The purpose of RE-VERSE AD was to evaluate the safety and efficacy of idarucizumab, a humanized Fab fragment with high-affinity binding for dabigatran [Glund S et al. Thromb Haemost. 2015].
In the multicenter, open-label, phase 3 RE-VERSE AD study, 300 patients taking dabigatran with uncontrolled bleeding or who require emergent surgery or procedure and received 2 infusions of idarucizumab 2.5 g within 24 hours of presentation will be enrolled [Pollack CV et al. N Engl J Med. 2015]. Patients are followed for 90 days. The authors have presented and published an interim analysis of the first 90 patients in this ongoing study.
The primary end point is the maximum percentage of reversal of anticoagulation by dabigatran, which is measured by dilute thrombin time (dTT) or ecarin clotting time (ECT) conducted within 4 hours post idarucizumab. The secondary end points include cessation of bleeding among patients with uncontrolled bleeding, hemostasis during an emergent procedure, posttreatment thrombosis within 90 days, mortality, time to complete anticoagulation reversal, duration of anticoagulation reversal, serial dabigatran levels, use of blood products and other supportive therapies, restart of antithrombotic therapy, development of antibodies against idarucizumab, and modified Rankin scores for intracranial hemorrhage.
Among the first 90 patients, the median age was 76.5 years, and median creatinine clearance was 58. The median patient-reported time since their last dose of dabigatran was 15.4 hours; 76% had an elevated dTT at baseline, and 90% had an elevated ECT at baseline.
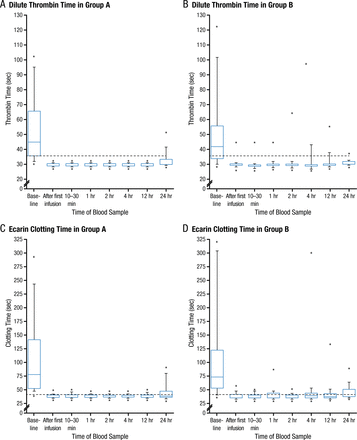
Among patients with uncontrolled bleeding or who required an emergent procedure, administration of idarucizumab resulted in a median maximum anticoagulation reversal within 4 hours of 100%, which was evident after the first infusion (Figure 1). The reversal of dTT and ECT was 100% (95% CI, 100% to 100%) and was sustained for at least 24 hours. Normalization of dTT occurred in 98% and 93% of patients with uncontrolled bleeding and those requiring emergent procedures, respectively, and ECT was normalized in 89% and 88% of patients. However, some patients experienced a reappearance of a low level of dabigatran after 24 hours. This is likely due to gradual redistribution of dabigatran from peripheral compartments, but it did not appear to have any clinical effect. The median time to bleeding cessation in patients with uncontrolled bleeding, in whom that end point could be assessed, was 11.4 hours. In patients who required an emergent procedure, normal intraoperative hemostasis was achieved in 92% of patients; none had markedly abnormal hemostasis.
Effect of Idarucizumab on Dabigatran-Mediated Anticoagulation Among Patients With Uncontrolled Bleeding (Panels A and C) and Those Who Require Emergent Procedures (Panels B and D)
The analyses included 51 patients who had serious bleeding (group A; Panels A and C) and 39 who required urgent surgery or intervention (group B; Panels B and D). Idarucizumab was administered in two infusions. Blood samples were obtained at baseline, after the first infusion, at 10 to 30 minutes after the administration of the second infusion, and at 1, 2, 4, 12, and 24 hours. Data are presented as box-and-whisker plots, in which the top and bottom of the rectangles indicate the 75th and 25th percentiles, respectively; the horizontal lines within the rectangles indicate the 50th percentile; the lines above and below the rectangles indicate the 90th and 10th percentiles, respectively; and the dots above and below the lines indicate the 95th and 5th percentiles, respectively. The dashed lines indicate the upper limit of the normal range for the tests.
From N Engl J Med, Pollack CV Jr et al., Idarucizumab for Dabigatran Reversal. Copyright © (2015) Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
Posttreatment thrombosis occurred in 5 patients; 1 case occurred within 72 hours after receiving idarucizumab, and 4 occurred 72 hours after idarucizumab administration. None of these patients were receiving antithrombotic therapy at the time of thrombosis. There were 18 deaths, all of which were associated with their presenting condition.
Dr Pollack stated that the study was open label and single arm because there are currently no approved treatment strategies for comparison in these patient populations. In addition, he stated that there were no safety concerns to date about idarucizumab, which led to complete and immediate anticoagulation reversal in up to 98% of patients. The study will continue.
- © 2015 SAGE Publications