Summary
Intracerebral hemorrhage (ICH), not related to trauma, is a significant cause of morbidity and mortality. Therapeutic options are few, and none are proven to reduce morbidity or mortality. This article discusses the epidemiology of ICH, use of neuroimaging to establish etiology and to stratify risk for nontraumatic ICH, genetic markers of ICH, as well as the the risk of ICH associated with statin therapy, nonsteroidal anti-inflammatory drugs, alcohol, selective serotonin reuptake inhibitors, and vitamin E.
- Ischemia
- Neuroimaging
- Ischemia
- Neuroimaging
- Neurology
Intracerebral hemorrhage (ICH), not related to trauma, is a significant cause of morbidity and mortality. Therapeutic options are few, and none are proven to reduce morbidity or mortality. Dawn Kleindorfer, MD, University of Cincinnati, Cincinnati, Ohio, USA, reviewed the epidemiology of ICH.
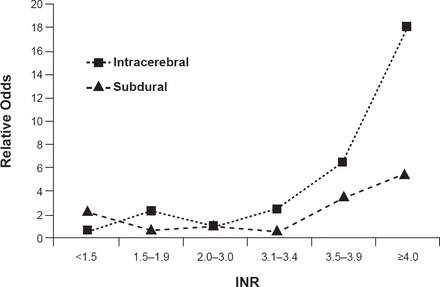
ICH represents ∼10% of all strokes [Go AS et al. Circulation 2014] and has a 43% mortality rate at 6 months. Only 12% of patients are left with minor or no disability. The primary injury is due mainly to the mass effect of the hematoma. Symptoms often progress as the hematoma grows or when there is a rupture of the blood into the ventricles. Secondary injury is mostly associated with the effects of the blood products such as edema or inflammatory reaction. Risk factors, which vary somewhat by location of the hematoma, include advancing age, hypertension (associated with 70% of ICH; primarily deep ICH), being black or Hispanic, use of warfarin, prior ischemic stroke, and possibly family history and alcohol use. Optimal use of warfarin for atrial fibrillation is an important factor for controlling ICH risk as international normalized ratio (INR) values ≥3.4 are associated with increased risk (Figure 1) [Fang MC et al. Ann Intern Med 2004]. Other possible risk factors like cholesterol and smoking need more confirming studies.
Warfarin and INR as ICH Risk Factors
INR=international normalized ratio.
Reproduced from Fang MC et al. Advanced Age, Anticoagulation Intensity, and Risk for Intracranial Hemorrhage Among Patients Taking Warfarin for Atrial Fibrillation. Ann Int Med 2004;141(10):745–752. With permission from the American College of Physicians.
The location of an ICH is important in terms of symptom severity, outcomes, and etiology. Brainstem ICH has the least probability of survival. Hemorrhage volume, baseline Glasgow Coma Scale score, and the presence of intraventricular extension are also predictors of outcome. Small differences in hemorrhage volume can make a big difference in outcome and mortality (ie, a ping pong ball-sized hemorrhage [28 mL] has a mortality rate of 19% compared with 20% to 55% for one the size of golf ball [41 mL]).
Mahmut Gurol, MD, MSc, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts, USA, discussed the use of neuroimaging to establish etiology and to stratify risk for nontraumatic ICH including hypertensive small vessel disease and cerebral amyloid angiopathy (CAA).
Noninvasive imaging of brain vasculature using computed tomography and magnetic resonance (MR) angiography can identify deep hemispheric ICH in hypertensive patients with nontraumatic hemorrhage. T2* weighted MRI images are useful for identifying microbleeds in cortico-, subcortical, and lobar micro- and macro-hemorrhages. Microbleeds are common in patients with ICH, particularly lobar ICH related to CAA. Their presence is related to all clinical symptoms/syndromes associated with underlying microvasculopathies such as cognitive impairment, ICH, and ischemic stroke. An increasing number of baseline hemorrhages can predict increased risk for subsequent cognitive impairment, loss of independence, or death [Greenberg SM et al. Stroke 2004].
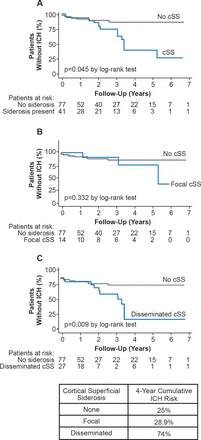
Sulcal siderosis is a focal subarachnoid hemorrhage in a cortical sulcus often associated with CAA. It can be identified with gradient recalled echo and susceptibility weighted MRI. Cortical superficial siderosis (particularly if disseminated) is a common and characteristic feature of CAA and increases the risk of recurrent ICH (Figure 2) [Charidimou A et al. Neurology 2013].
Cortical Superficial Siderosis and Risk of Intracerebral Hemorrhage in Cerebral Amyloid Angiopathy
cSS=cortical superficial siderosis; ICH=intracerebral hemorrhage.
Reproduced from Charidimou A et al. Cortical superficial siderosis and intracerebral hemorrhage risk in cerebral amyloid angiopathy. Neurology 2013;81(19);1666–1673. With permission from Lipincott Williams and Wilkins.
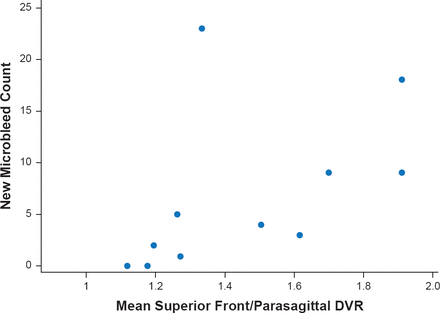
Dr. Gurol concluded with a discussion of use of positron emission tomography imaging with Pittsburgh compound B to predict CAA related hemorrhages. Mean distribution volume ratio in a superior frontal/parasagittal region of interest significantly (p=0.003) correlated independently with number of future hemorrhages in CAA subjects (Figure 3) [Gurol ME et al. Neurology 2012].
Pittsburgh Compound B PET Predicts CAA-Related Hemorrhages
DVR=distribution volume ratio; PET=positron emission tomography
Reproduced from Gural ME et al. Predicting sites of new hemorrhage with amyloid imaging in cerebral amyloid angiopathy. Neurology 2012;79(4):320–326. With permission from Lipincott Williams and Wilkins.
Genetic variations, such as those at apolipoprotein E (APOE) genes, are being identified that influence the risk of spontaneous ICH. In addition, two new loci have been identified that suggest a biological heterogeneity across ICH subtypes. Joan Montaner, MD, PhD, Hospital Vall d'Hebron, Barcelona, Spain, discussed genetic markers of ICH risk that may identify novel pathways and prevention strategies.
Results of a 2002 study showed that having a first-degree relative with an ICH is an independent predictor for deep ICH (OR, 6.3; 95% CI, 1.1 to 22) [Woo D et al. Stroke]. The influence of genetics is also supported by a recent study showing genetic variation having a substantial role in ICH risk, outcome, and hematoma volume [Devan WJ et al. Stroke 2013].
Single gene disorders that play a role in ICH have also been identified. For example, a mutation in the mouse Col4a1 gene, encoding procollagen type IV alphal, predisposes both newborn and adult mice to small vessel disease and hemorrhagic stroke [Gould DB et al. N Engl J Med 2006]. Among men, a polymorphism in betal-tubulin (TUBB1 Q43P) has been found to significantly increase (p=0.021) the risk of ICH and to be associated with an earlier age of occurrence (p=0.011). Carriers of the TUBB1 Q43P polymorphism have been found to display lower platelet reactivity towards collagen, but an increased risk of ICH [Navarro-Nunez L et al. Haematologica 2007].
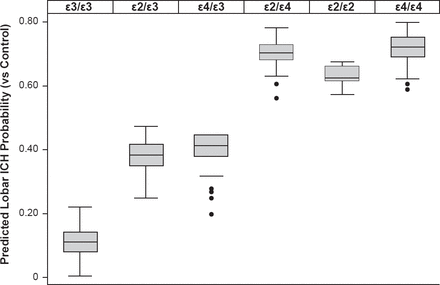
Variants at APOE have been associated with lobar (alleles ε2 and ε4) as well as deep (allele ε4) ICH at a genome-wide significance level. A stronger effect was noted when analysis was restricted to definite/probable CAA ICH (Figure 4) [Biffi A et al. Ann Neurol 2010].
Impact of Variants at APOE on ICH Probability
ICH=intracerebral hemorrhage.
Reproduced from Biffi A et al. Variants at APOE influence risk of deep and lobar intracerebral hemorrhage. Ann Neurol 2010;68(6):934–943. With permission from John Wiley & Sons, Inc.
APOE ε4 influences the risk of deep and lobar ICH in participants of both European ancestry and African-American descent. Carriers of APOE ε2 with lobar ICH have larger ICH volumes compared with noncarriers [Biffi A et al. Lancet Neurol 2011] and greater chance of ICH recurrence [Domingues-Montanari S et al. Neurobiol Aging 2011].
Hypertension is a noted risk factor for ICH and several hypertensive pressure-related alleles are associated with increased risk of deep ICH as well as with clinically identified hypertension [Falcone GJ et al. Stroke 2012].
The future of ICH genetics requires a collaborative effort and new genetic tools. A number of ongoing genome-wide studies are looking at the impact of genetics on ICH. One of these is the Ethnic/Racial Variations of Intracerebral Hemorrhage study [ERICH], a large, case-control study of ICH with particular emphasis on recruitment of minorities.
Philip Gorelick, MD, MPH, Michigan State University College of Human Medicine and Mercy Health Hauenstein Neurosciences, Grand Rapids, Michigan, USA, reviewed the risk of ICH associated with statin therapy, nonsteroidal anti-inflammatory drugs (NSAIDs), alcohol, selective serotonin reuptake inhibitors (SSRIs), and vitamin E.
His conclusions regarding these agents were as follows:
Statins: In the SPARCL trial, there was only a slight excess of hemorrhagic stroke with atorvastatin versus placebo (2.3% vs 1.4%). If a risk exists, the absolute magnitude is likely to be small and outweighed by the advantages of statins for reduction of major vascular events. However, it remains unclear whether statins should be avoided for patients with a history of ICH, especially lobar.
NSAIDs: Concerns with NSAIDs center on risk for thrombosis and gastrointestinal bleeding but there is no apparent ICH signal. Moderate to high doses of aspirin (>1225 mg/week spread over ≥3 doses) carry a high risk of ICH (OR, 3.05; 95% CI, 1.02 to 9.14; p=0.047). Nonaspirin NSAIDS are not a risk for ICH hospitalization.
Alcohol: There is a positive linear association with the number of drinks and the occurrence of ICH. Possible mechanisms include hypertension, coagulation disturbances, and activation of adrenergic receptors.
SSRIs: SSRIs may be linked to ICH but the absolute risk is small (one additional intracerebral bleeding episode/10000 persons treated for 1 year). This may be enhanced, however, by the use of oral anticoagulants or history of ICH (particularly lobar).
Vitamin E: There may be an increased risk of ICH but the absolute effects are small and vitamin E may reduce ischemic stroke risk. Vitamin E should be used with caution.
The editors would like to thank the many members of the International Stroke Conference presenting faculty who generously gave their time to ensure the accuracy and quality of the articles in this publication.
- © 2014 MD Conference Express®