Summary
This article discusses updates in arrhythmia technologies including the computational modeling of arrhythmia, left atrial appendage occlusion or exclusion devices, new noninvasive ablation technologies, as well as new lead designs, leadless systems, and sensors.
- Interventional Techniques & Devices
- Arrhythmias
- Imaging Modalities
- Interventional Techniques & Devices
- Arrhythmias
- Cardiology & Cardiovascular Medicine
- Imaging Modalities
Natalia A. Trayanova, PhD, Johns Hopkins University, Baltimore, Maryland, USA, discussed the computational modeling of arrhythmia, with emphasis on clinical applications. Virtual electrophysiology based on magnetic resonance imaging (MRI) translates the information from patient images to construct functional models of the heart, geared toward better understanding how to provide heart treatment for various arrhythmia disorders. One of the projects involves predicting the optimal targets for ventricular tachycardial ablation, in which heart mapping is helping to identify the best sites for ablation. The goals include reducing the risks associated with the invasive procedure and shortening the procedure time.
Patients referred for ablation receive a pre-ablation MRI scan that generates 2-dimensional (2D) image slices. The slices of information, including the region of the infarct scar and the immediately surrounding semiviable tissue, are computationally melded together to generate a 3D model of the heart that also incorporates the estimated orientation of the cardiac fibers.
The process is patient specific. All possible arrhythmias that could occur based on the pathology are modeled, and the optimal ablation target is determined noninvasively. Identifying the optimal target of ablation allows the swift and precise delivery of ablation, smaller ablation lesions, and improved patient tolerance of the procedure.
Shephal Doshi, MD, Pacific Heart Institute, Santa Monica, California, USA, discussed left atrial appendage occlusion or exclusion devices. The devices are designed to trap blood clots before they can exit. Currently, no such device has received United States Food and Drug Administration (FDA) approval for stroke prevention.
The Watchman device has been tested in >2000 patients in 6 clinical trials, including several randomized clinical trials. The feasibility of the Amplatzer cardiac plug (ACP) device is apparent based on 31 implants. A pivotal trial was closed with 100 subjects enrolled and 65 device implants performed. Clinical trials are beginning for the remaining devices. The Wavecrest device has been implanted in more than 200 patients worldwide and is being tested in a US-based pivotal trial. Experience to date with the Lambre device has involved 80 implants in Europe and Asia. CE Mark (which indicates that the product meets the relevant requirements for approval) submission is pending for the Lambre device for European marketing, but the other 3 have received the CE Mark.
The aforementioned devices position the sheath to the desired location in the left atrium before deployment. The final device, the Lariat epicardial snare suture device, differs in design, with a lariat being used to draw together and close the appendage. The Lariat device has been extensively used, with more than 2300 implants worldwide and a use rate of 80 to 100 cases each month in the United States. It has FDA approval for soft-tissue approximation. Experience in other countries has been limited.
Efforts to improve these devices are focusing on four aspects (Table 1).
Areas Requiring Improvement
Electrocardiographic imaging (ECGI) allows noninvasive mapping that can guide ablation. ECGI approaches include body surface potential mapping, noncontrast computed tomography, and computational mapping of sinus rhythm [Cuculich PS et al. Circulation 2010]. The drivers of persistent AF have been mapped noninvasively in real time in more than 300 cases in Europe using the ecVUE platform. The technology does not yet have FDA approval.
Noninvasive mapping of ventricular fibrillation (VF) is challenging but possible, and it has revealed the important role of the Purkinje network in triggering (and perhaps sustaining) VF [Haissaguerre M et al. Lancet 2002]. A mapping procotol using both invasive and noninvasive approaches may be useful in treating triggers of VF [Wang Y et al. Sci Trans Med 2011]. Mapping can potentially be extended beyond the direction of ablation to better understand the pathogenesis of arrhythmia.
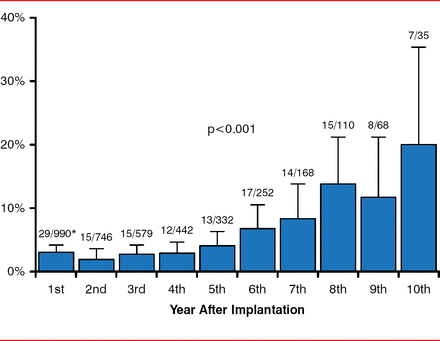
Andrea M. Russo, MD, Cooper University Hospital, Camden, New Jersey, USA, considered new lead designs, leadless systems, and sensors. The lead of the implantable cardioverter defibrillator (pacemaker) system is the weakest link [Kleemann T et al. Circulation 2007], with lead failure (most commonly due to the insulator component) increasing steadily with time (Figure 1).
Annual Rate of Defibrillation Lead Defects
Reproduced from Kleemann T et al. Annual Rate of Transvenous Defibrillation Lead Defects in Implantable Cardioverter-Defibrillators Over a Period of >10 Years. Circulation 2007;115:2474–2480. With permission from Lippincott Williams and Wilkins.
The durability and longevity of ICD leads present challenges, especially in younger patients who tend to be more active and will require multiple leads during their life, and also because the lead is exposed to >30 million cardiac contractions annually. Extraction of leads can be hindered by fibrosis that occurs at the electrode-myocardium interface. Improvement efforts include the use of an electrically inert, expanded polytetraflouroethylene polymer coating that permits the passage of electrically conductive fluid but not blood and tissue cells [Hackler JW. Heart Rhythm 2010].
Another innovation is a lead system that is completely subcutaneous and does not require cardiac electrodes. FDA approval of the Subcutaneous Implantable Defibrillator (S-ICD) System was granted in 2012. An intravascular defibrillator has shown promise in a small trial involving 10 patients with ischemic cardiomyopathy and left ventricular ejection fraction ≤35% [Neuzil P et al. Heart Rhythm 2014], as has an ultrasound-based cardiac stimulation system [Auricchio A et al. Europace 2013]. Leadless cardiac pacing using a small, self-contained pacemaker was also investigated in the LEADLESS trial [Reddy VY et al. Circulation 2014]. The device proved safe and feasible for use.
These innovations still need to pass the tests of greater clinical scrutiny. For the present, lead complications including infections and reliability remain vexing issues.
Finally, Paul J. Wang, MD, Stanford University School of Medicine, Stanford, California, USA, discussed new noninvasive ablation technologies, including laser, cryofreezing, ultrasound and high-frequency focused ultrasound, radiofrequency, and mapping. Such technologies seek to minimize catheter-associated risks of ablation, including stroke, perforation, pulmonary vein stenosis, esophageal fistula, and vascular events.
A meta-analysis reported the efficacy and safety of cryoballoon ablation, with a success rate exceeding 98% [Andrade JG et al. Heart Rhythm 2011]. In the Sustained Treatment of Paroxysmal Atrial Fibrillation study [STOP AF; Packer DL et al. J Am Coll Cardiol 2013], the use of cryoballoon ablation yielded an AF-free rate at 12 months of 69.9% (114/163) versus 7.3% (6/82) for antiarrhythmic drug therapy in AF patients refractory to prior treatment (p<0.001). Refinements in the cryoballoon equipment have produced more uniform, distal cooling while retaining the technique's simplicity, relatively rapid procedure time, efficacy, and safety.
VytronUS recently introduced a low-intensity collimated ultrasound device, which is purported to deliver more targeted ablation to lesions of any shape. Modeling of the heart, including the region of ablation, based on information acquired from computed tomography is improving the specificity and outcome of ablation.
These and other technologies, including the use of positron emission tomography, appear to decrease ablation-associated risks, with a shorter learning curve required for optimal performance of the techniques. Data for persistent, long-standing AF and very dilated or scarred atria are, however, lacking. With the promise of new technology, arrhythmia detection, monitoring, and interventions are improving. The comparative effectiveness of these competing devices will be informative for health systems that are struggling to balance improving patient outcomes with the exploding cost to afford expansion of this technology into routine arrhythmia practice.
- © 2014 MD Conference Express®