Summary
Defibrillation testing does not improve the efficacy of first shock after implantable cardioverter defibrillator (ICD) placement, nor does it decrease all-cause mortality. This article presents data from the Shockless Implant Evaluation trial [SIMPLE; NCT00800384].
- Arrhythmias
- Interventional Techniques & Devices
- Cardiology Clinical Trials
- Cardiology & Cardiovascular Medicine
Defibrillation testing does not improve the efficacy of first shock after implantable cardioverter defibrillator (ICD) placement, nor does it decrease all-cause mortality. Jeffrey S. Healey, MD, McMaster University, Hamilton, Ontario, Canada, presented data from the Shockless Implant Evaluation trial [SIMPLE; NCT00800384].
Although defibrillation testing is typically performed when an ICD is implanted, it can lead to serious complications (eg, refractory ventricular fibrillation/ventricular tachycardia) or death. In addition, the efficacy and safety of defibrillation testing is controversial, and it has not showed improved outcomes. The SIMPLE trial tested the hypothesis that intraoperative defibrillation testing is noninferior to no defibrillation testing following ICD implantation. In addition, it was expected that no testing would decrease the rate of serious perioperative complications at 30 days and would not increase all-cause mortality.
In this multicenter single-blind trial, 2500 patients undergoing an initial transvenous ICD implantation were randomly assigned to undergo defibrillation testing or no defibrillation testing. Exclusion criteria included a planned right-sided implant, ICD pulse generator replacement, and placement on the active cardiac transplant list. The mean age was 62 years, with 81% male and 64% to 66% with coronary artery disease. Other conditions included dilated cardiomyopathy (31% to 33%), hypertrophic cardiomyopathy (3% to 4%), and long QT, Brugada, or catecholaminergic polymorphic ventricular tachycardia (2%); 50% to 52% had previously undergone percutaneous coronary intervention or coronary artery bypass. In addition, the mean left ventricular ejection fraction was 32%, and 23% of patients had a history of atrial fibrillation. The mean follow-up was 3.1 years.
The primary efficacy outcome was a composite of ineffective first appropriate clinical shock or arrhythmic death. Secondary safety outcomes included rate of serious perioperative complications at 30 days and all-cause mortality. For protocol adherence, 4.5% in the no-defibrillator testing arm and 8.5% in the defibrillator testing arm had clinical shock programming >31 J.
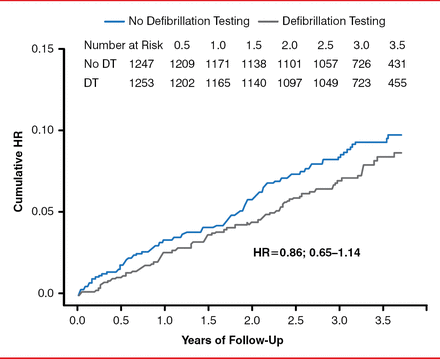
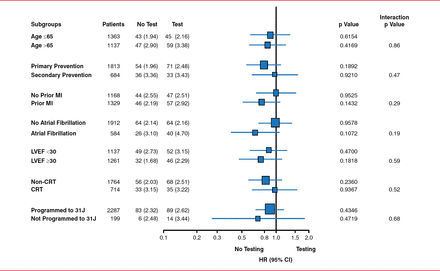
Patients who did not undergo defibrillation testing had similar rates of ineffective first appropriate clinical shock or arrhythmic death when compared to patients treated with defibrillation testing (HR, 0.86; 95% CI, 0.65–1.14; p=0.0001 noninferiority; Figure 1). In addition, the first appropriate ICD shock was likely to be successful in the no-defibrillation testing arm as compared with the defibrillation testing arm (p=0.08). However, there was no significant difference in the first shock success for monomorphic or polymorphic ventricular tachycardia. There was no significant difference in all-cause mortality between the study arms. Similar findings were seen across the other subgroups presented (Figure 2).
Effect of No Defibrillator Testing During Implantable Cardioverter Defibrillator Implantation on Failed Appropriate Shock or Arrhythmic Death
DT=defibrillator testing.
Reproduced with permission from JS Healey, MD.
Subgroup Analysis of the Primary Outcome in the SIMPLE Trial
CRT=cardiac resynchronization therapy; LVEF=left ventricular ejection fraction; MI=myocardial infarction.
Reproduced with permission from JS Healey, MD.
As compared with the no-defibrillation therapy arm, significantly more patients in the group treated with defibrillation therapy had death, stroke, non-central nervous system systemic embolism, pulmonary embolism, myocardial infarction, heart failure, intraoperative hypotension, need for chest compression, or nonelective intubation (4.5% vs 3%; p=0.047).
Dr. Healey concluded that the SIMPLE trial did not suggest that defibrillation testing improves the outcomes of patients undergoing ICD implantation.
- © 2014 MD Conference Express®