Summary
Proper function of the leptin–melanocortin axis is important in regulating food intake and maintaining energy homeostasis. Therefore, mutations that affect the leptin–melanocortin axis result in several known syndromes that result in an obesity phenotype. Recent research has further identified important components of this axis that may result in childhood-onset obesity as discussed in this article.
- obesity
- obesity
- pediatric nutrition
- prevention & screening
Proper function of the leptin–melanocortin axis is important in regulating food intake and maintaining energy homeostasis. Therefore, mutations that affect the leptin–melanocortin axis result in several known syndromes that result in an obesity phenotype. Recent research has further identified important components of this axis that may result in childhood-onset obesity.
Christian Vaisse, MD, PhD, University of California San Francisco, San Francisco, California, USA, discussed the role of primary cilia function in pediatric obesity. Several syndromes caused by genetic aberrations can result in pediatric obesity, including the Bardet–Biedl, Prader–Willi, Alstrom, Borjeson–Forssman–Lehman, Cohen, and Carpenter syndromes. For Bardet–Biedl syndrome, which can result in polydactyly, retinal degeneration, polycystic kidney disease, and obesity, > 12 genes have been identified, and these encode different proteins that play a role in the function of primary cilia.
Primary cilia are single, immotile sensory organelles that are present on multiple cell types, and their function is to transfer a variety of information from the extracellular environment to the cell. Ciliopathies have been implicated in a variety of disorders, including polycystic kidney disease, infertility, polydactyly, liver fibrosis, cerebellar defects, retinal degeneration, and heterotaxia. New data suggest that a ciliopathy may also result in obesity.
In animal models, ubiquitous impairment of primary cilia resulted in obesity. Furthermore, ablation of the primary cilia within differentiated neurons resulted in obesity in mice [Davenport JR et al. Curr Biol 2007]. These data suggest that a receptor is required at the level of the primary cilium in differentiated neurons for long-term energy homeostasis.
In the leptin–melanocortin axis in the hypothalamus, leptin produced by adipocytes causes a signaling cascade that results in the activation of the melanocortin 4 receptor (MC4R) protein, which in turn signals to stop food intake. This pathway led to the hypothesis that primary cilia are important for MC4R function and that impaired MC4R function causes ciliopathies that result in obesity. In vitro data have demonstrated that all neurons that express MC4R also have primary cilia and that MC4R localizes to the primary cilia. In addition, an intracranial infusion of Melanotan (MT) II into mice resulted in decreased food intake. Primary cilia ablation resulted in body weight gain and impaired MC4R function. In human cells, the Pro78Leu, Pro230Leu, and Arg236Cys mutations in MC4R caused impaired cilia expression.
Dr. Vaisse concluded by highlighting the importance of MC4R expression for proper primary cilia function, and by stating that impaired cilia function, frequently caused by MC4R dysfunction, can result in obesity in animal models. Therefore, MC4R mutations in humans may cause a genetic predisposition for obesity.
Joan C. Han, MD, National Institutes of Health, Bethesda, Maryland, USA, presented the role of brain-derived neurotropic factor (BDNF) in multiple forms of pediatric obesity. BDNF is a protein that is widely expressed in the nervous system and is important in the development of the nervous system during embryogenesis, as well as postnatal synaptic plasticity and neuronal survival [Pruunsild P et al. Genomics 2007].
Based on animal data, BDNF is downstream of the leptin–proopiomelanocortin signaling pathway, in which activation of BDNF inhibits food intake [Wisse BE, Schwartz MW. Nat Neurosci 2003]. In addition, haploinsufficiency of BDNF in mice results in hyperphagia and obesity. In humans, BDNF haploinsufficiency can cause an obesity subphenotype of WAGR syndrome, a rare disorder that is characterized by the classic clinical features of Wilms tumor, aniridia, genitourinary anomalies, and cognitive impairment. A subset of patients with WAGR syndrome also develop childhood-onset obesity. In patients with WAGR syndrome, the presence of BDNF haploinsufficiency is associated with higher body mass index (BMI) Z-score, childhood obesity, and hyperphagia [Han JC et al. N Engl J Med 2008]. Analysis of the BDNF gene in patients with WAGR syndrome found that a deletion of the first 3 exons of BDNF is sufficient to cause the obesity subphenotype. In the general population, genome-wide association studies have consistently identified the BDNF gene locus as a region associated with obesity [Thorleifsson G et al. Nat Genet 2009], suggesting that BDNF insufficiency may have a role in common forms of obesity as well.
Approaches for treating BDNF insufficiency focus on small-molecule agonists of the BDNF receptor, TrkB, or other mechanisms for increasing endogenous levels, because most peripherally administered BDNF does not cross the blood–brain barrier; one such pharmacologic agent that shows promise is amitriptyline [Chadwick W et al. PLoS One 2011].
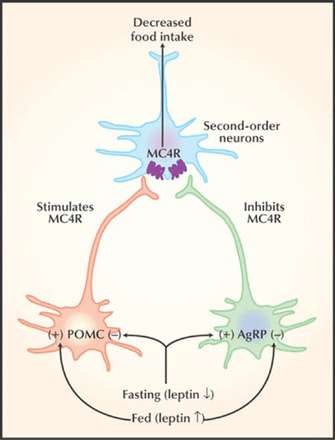
Joseph A. Majzoub, MD, Boston Children's Hospital, Boston, Massachusetts, USA, described the association of melanocortin receptor 2 accessory protein (MRAP2) and pediatric obesity. Recent advances in signaling pathways that regulate food intake have shed light on genetic causes of pediatric obesity. These pathways involve leptin, which ultimately regulates melanocortin 4 (MC4R), mutations that appear to result in impaired inhibition of food intake, thus resulting in increased food intake (Figure 1).
Signaling Pathway That Affects Food Intake
Reproduced from O'Rahilly S et al. Melanocortin receptors weigh in. Nature Medicine (2004). 10.4:351–352. With permission from Nature Publishing Group.
MRAP2 is an interacting partner of the MC4R receptor. Interestingly, mouse MRAP2 has recently been demonstrated to interact with MC4R in vitro, which appears to play a role in mammalian energy balance [Asai M et al. Science 2013]. In addition, MRAP2 knockout mice have greater body weight that progressively increases throughout time compared with their wild-type and heterozygous counterparts, despite similar fecal energy output, energy expenditure, and locomotor activity. In obese humans, in MRAP2, located on chromosome 6, several amino acid–changing mutations have been identified, including 1 in exon 2 and 1 in exon 4 [Asai M et al. Science 2013]. Although further human studies are required, these data suggest that mutations in MRAP2 may play a role in obesity.
- © 2014 MD Conference Express®