Summary
Current management of gout is suboptimal, and patients often do not achieve serum uric acid (SUA) targets with allopurinol monotherapy. This article discusses the results of 2 replicate phase 3 studies known as Combining Lesinurad With Allopurinol in Inadequate Responders [CLEAR 1 and CLEAR 2; NCT01510158 and NCT01493531, respectively] conducted in symptomatic adults with gout.
- Inflammatory Disorders Rheumatology Clinical Trials
- Inflammatory Disorders
- Rheumatology
- Rheumatology Clinical Trials
More patients with gout who were randomized to the novel selective uric acid (UA) reabsorption inhibitor lesinurad in combination with allopurinol reached their serum uric acid (SUA) target compared with patients randomized to allopurinol plus placebo.
Kenneth G. Saag, MD, University of Alabama at Birmingham, Birmingham, Alabama, USA, reported the results of 2 replicate phase 3 studies known as Combining Lesinurad With Allopurinol in Inadequate Responders [CLEAR 1 and CLEAR 2; NCT01510158 and NCT01493531, respectively] conducted in symptomatic adults with gout. Current management of gout is suboptimal, and patients often do not achieve SUA targets with allopurinol monotherapy [Zhang W et al. Ann Rheum Dis. 2006; Becker MA et al. N Engl J Med. 2005]. Lesinurad inhibits the UA transporter 1, increasing UA excretion and thereby lowering SUA.
CLEAR 1 (US patients; n = 603) and CLEAR 2 (global study; n = 610) were 12-month multicenter, randomized, placebo-controlled studies in which lesinurad 200 and 400 mg QD, added to a medically appropriate stable dose of allopurinol (at least 300 mg QD, and at least 200 mg QD for those with moderate renal impairment), was compared with placebo plus allopurinol. Eligible patients had SUA levels ≥ 6.5 mg/dL at the screening visit and ≥ 6.0 mg/dL at the day 7 visit, and had ≥ 2 gout flares in the 12 months prior to randomization.
In both studies, patients were primarily white, and ≥ 90% were male. Mean ages were 51.9 years in CLEAR 1 and 51.2 years in CLEAR 2, and mean years since gout diagnosis were 11.84 and 11.53, respectively. Ninety-one percent of patients in CLEAR 1 and 84% in CLEAR 2 were receiving allopurinol 300 mg/d at screening, and 14% and 23%, respectively, had tophi. Baseline SUA levels were 6.94 and 6.90 mg/dL in CLEAR 1 and CLEAR 2, respectively.
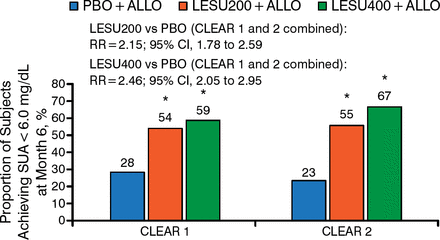
The primary efficacy outcome—the proportions of patients achieving their SUA target at 6 months—was significantly greater for lesinurad 200 or 400 mg in combination with allopurinol compared with allopurinol alone in both CLEAR 1 and CLEAR 2 (Figure 1).
Percentage of Patients Achieving Serum Uric Acid < 6.0 mg/dL at Month 6a
Relative risks are post hoc calculations, not adjusted for randomization stratification factors.
ALLO, allopurinol; CLEAR, Combining Lesinurad With Allopurinol in Inadequate Responders; LESU, lesinurad; PBO, placebo; SUA, serum uric acid.
*P < .0001 vs PBO + ALLO arm.
aNonresponder imputation. If a patient drops out or there are missing data, that patient is assumed to be a nonresponder, regardless of whether they were responding to treatment at the last measurement.
Reproduced with permission from KG Saag, MD.
The mean number of gout flares requiring treatment (end of month 6 through month 12) was not significantly different between treatment groups. The proportion of patients with complete tophus resolution by month 12 was also not significantly different between groups.
Lesinurad was generally well tolerated. At the 200-mg dose, the number of treatment-emergent adverse events was comparable between lesinurad plus allopurinol and placebo plus allopurinol. Serum creatinine increases ≥ 1.5 times were more common with lesinurad compared with placebo, with a high of 15.9% in CLEAR 1 patients treated with 400 mg of lesinurad in combination with allopurinol. The vast majority of cases of serum creatinine elevations resolved without interrupting study medication.
There were few serious renal-related adverse events (AEs), with no difference in incidence between patients randomized to lesinurad 200 mg plus allopurinol and placebo plus allopurinol. The incidence of kidney stones was comparable between lesinurad 200 mg plus allopurinol and placebo plus allopurinol. The incidence of renal-related AEs and kidney stones was higher with lesinurad 400 mg plus allopurinol.
Combination therapy with lesinurad and allopurinol may represent a future treatment option for gout patients on allopurinol who warrant additional therapy, Dr Saag concluded.
- © 2014 MD Conference Express®