Summary
For patients with breast cancer who undergo primary surgery, sentinel lymph node biopsy (SLNB) is the standard staging procedure to determine the axillary status when the patient is clinically node negative [D'Angelo-Donovan DD et al. Surg Oncol 2012]. However, for patients who undergo neoadjuvant chemotherapy, the optimal role and timing of SLNB is still unclear. This article presents final results from the prospective German, multi-institutional Sentinel Neoadjuvant [SENTINA] trial.
- Oncology Clinical Trials
- Breast Cancer
- Adjuvant/Neoadjuvant Therapy
- Radiology
For patients with breast cancer who undergo primary surgery, sentinel lymph node biopsy (SLNB) is the standard staging procedure to determine the axillary status when the patient is clinically node negative [D'Angelo-Donovan DD et al. Surg Oncol 2012]. However, for patients who undergo neoadjuvant chemotherapy (NACT), the optimal role and timing of SLNB is still unclear. Thorsten Kuehn, MD, Klinikum Esslingen, Esslingen, Germany, presented final results from the prospective German, multi-institutional Sentinel Neoadjuvant [SENTINA] trial.
The SENTINA trial aimed to evaluate a specific algorithm for the timing of a standardized SLNB procedure and provide data on sentinel lymph node detection rates prior to and after NACT. In addition, the trial assessed false-negative rates for patients who convert from cN1 to cN0 status following chemotherapy and determined factors that might influence detection rates and false-negative rates.
Patients (n=1737) at 103 institutions were distributed among 4 treatment arms according to clinical axillary staging before and after chemotherapy. Arms A and B included patients with cN0 status who underwent SLNB prior to primary systemic therapy. If the sentinel lymph node was negative histologically, no further axillary surgery was performed after primary systemic therapy and the patient was categorized in Arm A. If the sentinel lymph node was positive histologically, a second SLNB and axillary dissection was performed after primary systemic therapy, and the patient was categorized in Arm B. Arms C and D included patients with cN1 status who underwent no axillary surgery prior to primary systemic therapy. Patients who converted to cN0 after primary systemic therapy underwent SLNB and axillary dissection, and were categorized as Arm C; patients who remained cN1 status after primary systemic therapy underwent classical axillary dissection and were categorized as Arm D.
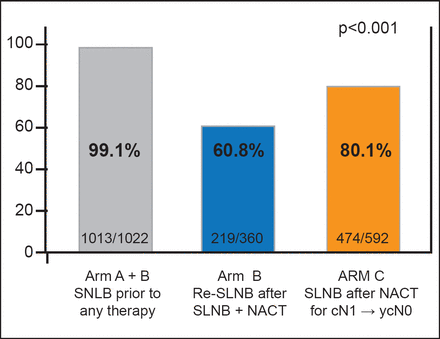
The SLNB detection rate was 99.1% (1013/1022) before primary systemic therapy for Arms A and B, 80.1% (474/592) after primary systemic therapy for Arm C, and 60.8% (219/360) for Arm B after prior SLNB and primary systemic therapy (p<0.001; Figure 1). In Arm B, from 219 patients with a detected sentinel lymph node following primary therapy, 29.2% (64) had a positive axillary status and 70.8% (155) had a negative axillary status. The sentinel lymph node false-negative rate for Arm B was 51.6% (33; 95% CI, 38.7 to 64.2). In Arm C, after primary systemic therapy, 47.7% (226/474) of patients with a detected sentinel lymph node had a positive axillary status and 52.3% (248/474) had a negative axillary status. The sentinel lymph node false-negative rate for Arm C was 14.2% (32 patients; 95% CI, 9.9 to 19.4).
Sentinel Lymph Nodes Detected and Removed.
NACT=neoadjuvant chemotherapy; SLNB=sentinel lymph node biopsy.
Reproduced with permission from T Kuehn, MD.
According to Prof. Kuehn, the sentinel lymph node detection rate is excellent for patients who receive SLNB prior to systemic therapy. However, the detection rate for repeated SLNB is “unacceptable.” Previous local and systemic treatment significantly impairs the tracer uptake and detection rate. Prof. Kuehn said, “SLNB as a diagnostic procedure is not a reliable tool in patients who convert under neoadjuvant chemotherapy from cN1 to cN0 compared with SLNB in primary surgery.”
- © 2013 MD Conference Express®