Summary
Disodium ethylene diamine tetra acetic acid (EDTA) binds divalent cations and permits renal excretion. The data for chelation have been mixed with some case reports and case series reporting benefit and other studies suggesting no benefit or even harm, particularly when rapid infusions cause hypocalcemia. This article reports the results of the Trial to Assess Chelation Therapy [TACT; NCT00044213].
- Cardiology Clinical Trials
- Myocardial Infarction
Disodium ethylene diamine tetra acetic acid (EDTA) binds divalent cations and permits renal excretion. A 1956 study reported improvement of angina with disodium EDTA [Clarke CN et al. Am J Med Sci 1956]. By 2007, its use had increased to more than 100,000 patients in the United States. The data for chelation have been mixed with some case reports and case series reporting benefit and other studies suggesting no benefit or even harm, particularly when rapid infusions cause hypocalcemia. Gervasio A. Lamas, MD, Mount Sinai Medical Center, Miami Beach, Florida, USA, reported the results of the Trial to Assess Chelation Therapy [TACT; NCT00044213].
The TACT trial enrolled 1708 patients from 2003 through 2010 [Lamas GA et al. Am Heart J 2012]. Patients were eligible if they were aged ≤50 years, had a myocardial infarction (MI) at least 6 months prior to enrollment, and creatinine ≤2.0 mg/dL. They were randomly assigned to chelation therapy or placebo, and high-dose vitamins or placebo in a 2×2 factorial design. The patients were scheduled to receive 40 infusions of at least 3 hours each given as 30 weekly infusions and followed by 10 maintenance infusions 2 to 8 weeks apart. The chelation infusions consisted of 3 g disodium EDTA adjusted downward based on estimated glomerular filtration rate.
The primary endpoint was a composite of death, MI, stroke, coronary revascularization, and hospitalization for angina. Data were analyzed according to the intention-to-treat principle. Because of multiple reviews of the interim data by the Data and Safety Monitoring Board, the final level of statistical significance was p<0.036.
Sixty-five percent of patients received all 40 infusions, and 76% received at least 30 infusions. A total of 79 patients (38 chelation, 41 placebo) discontinued infusions due to adverse events (AEs). Short infusions were administered in 611 instances. Four unexpected severe AEs possibly or definitely related to study therapy occurred—2 in the placebo arm (1 death) and 2 in the chelation arm (1 death).
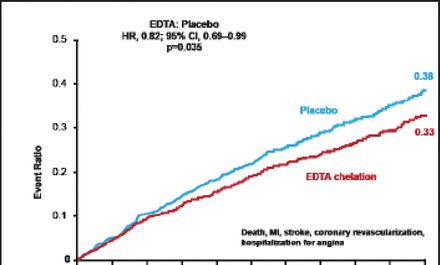
The primary endpoint event rate was significantly reduced in patients receiving chelation versus placebo (26.5% vs 30.0%; HR, 0.82; 95% CI, 0.69 to 0.99; p=0.035; Figure 1). There was directional consistency among the components of the primary endpoint in favor of chelation versus placebo (Table 1).
Components of the Primary Endpoint.
Primary Endpoint Results.
Reproduced with permission from GA Lamas, MD.
Subgroup analyses suggested that chelation was superior to placebo in patients with anterior MI (HR, 0.63; 95% CI, 0.47 to 0.86; interaction p=0.03) and diabetes (HR, 0.61; 95% CI, 0.45 to 0.83; interaction p=0.02).
Limitations of the TACT trial include the modest statistical significance, with the upper CI of the HR for the primary endpoint of 0.99, missing data (17% of patients withdrew consent), and revascularization being the most common efficacy event.
Dr. Lamas concluded that, within the safety net provided by TACT, chelation therapy appears to be safe. The 10-component disodium EDTA chelation and ascorbate regimen demonstrated some evidence of a potentially important treatment signal in post-MI patients already on evidence-based therapy. The TACT trial results are unexpected and additional research is needed to confirm or refute the results and explore possible mechanisms of benefit.
Though the results of the TACT trial are interesting, they should be interpreted with caution as Elliot Antman, MD, Chairman of the AHA Scientific Sessions 2012, Brigham and Women's Hospital, Boston, Massachusetts, USA, pointed out in a formal statement: “As intriguing as the results are, they're unexpected and should not be interpreted as an indication to adopt chelation therapy into clinical practice. Much more information is needed about which elements of the complex infusion mixture might provide benefit; the marked discordance between the observed treatment effect in diabetics versus nondiabetics needs to be understood….TACT raises more questions that must be answered before we are ready to act on the observations that were reported.”
- © 2012 MD Conference Express®