Summary
This article addresses how technological advances have clarified the pathophysiology of both transient and permanent brain ischemia.
- Neurology
- Episodic & Paroxysmal Disorders
- Ischemia
- Neuroimaging
Gregory W. Albers, MD, Stanford University Medical Center, Palo Alto, California, presented the David G. Sherman Lecture, which addressed how technological advances have clarified the pathophysiology of both transient and permanent brain ischemia.
Two years ago, the American Heart Association endorsed a new definition of transient ischemic attack (TIA), which defined TIA as a transient episode of neurological dysfunction that was caused by focal brain, spinal cord, or retinal ischemia, without acute infarction [Easton JD et al. Stroke 2009]. Unlike previous definitions, this concept does not refer to an arbitrary time limit for symptoms to resolve, and it emphasizes the absence of tissue injury.
On a recent visit to Japan, Dr. Albers conducted an informal survey and found that many clinicians have yet to accept this new definition; he also identified some potential misperceptions. The most commonly stated reason for lack of endorsement was the perception that the new definition does not affect patient management. Dr. Albers questioned this conclusion and wondered if the true clinical utility of the new definition had been made clear. “Knowing whether there is a small ischemic lesion in the brain of a patient who presents with transient ischemic symptoms is extremely beneficial,” Dr. Albers said. Recently, Giles and colleagues [Giles MF et al. Stroke 2010] reported that incorporating brain imaging evidence of infarction into the Age, Blood pressure, Clinical features, Duration 2 (ABCD2) score (Table 1) significantly improves the prediction of 7-day stroke risk. After adjustment for ABCD2 score, the odds ratio for a positive diffusion-weighted imaging (DWI) lesion was 14.9 (95% CI, 7.4 to 30.2; p<0.001). Adding acute infarction into the ABCD2 score dramatically improved the predictive power of this commonly used score. Furthermore, new data from the same research group [Giles et al. Under review] show a direct relationship between a positive DWI and increasing ABCD2 score for predicting stroke risk. This relationship is not apparent in patients with a negative DWI, who appear to have an extremely low stroke risk (eg, <1% at 7 days) regardless of ABCD2 score. A positive DWI provides evidence of a true ischemic event in these patients. Dr. Albers speculated that many patients with a negative DWI may have suffered a nonvascular event (TIA mimic) rather than a true TIA. Building on the new definition of TIA, there is a need for even further implementation of technology, such as perfusion-weighted MRI and CT perfusion, to help differentiate transient ischemic events from other conditions that can mimic the clinical appearance of a TIA [Mlynash M et al. Neurology 2009].
ABCD2 Score.
Imaging technology is also changing our understanding of the relationship between tissue salvageability and time in acute stroke patients. Baseline DWI is an accurate method for establishing the volume and location of the early ischemic core, and DWI can identify salvageable penumbra. In patients with early, complete reperfusion, DWI can predict about 90% of the final infarct volume with a small false-positive rate. Although reversibility of DWI lesions has been reported, recent clinical data have clarified that reversal is rare and typically involves only a small volume of tissue. Animal stroke studies have shown that normalization of the DWI does not necessarily imply tissue salvage; brain regions with both transient and complete reversal on imaging have been associated with microscopic evidence of structural damage to neurons [Ringer TM et al. Stroke 2001; Li F et al. Stroke 2000].
Stroke clinicians have long sought the ability to identify regions of critical hypoperfusion quickly and accurately. When appropriately processed, perfusion-weighted imaging (PWI) can identify hypoperfusion in acute ischemic stroke and reliably detects the penumbra using time-based thresholds [Takasawa M et al. Stroke 2008]. Recent studies have shown that the volumes of penumbral tissue identified with appropriate PWI thresholds match PET and xenon CT measures with high sensitivity and specificity [Zaro-Weber O et al. Stroke 2010]. If it is not reperfused rapidly, ischemic tissue with a delay in contrast arrival (Tmax delay) of ≥6 sec is associated with reliable lesion enlargement between the initial and follow-up scans and has been set as the penumbral threshold for several new trials.
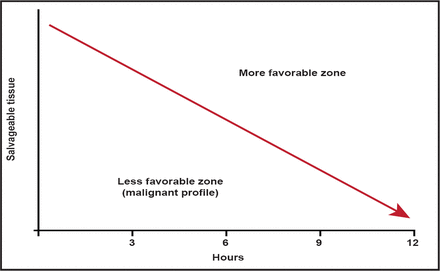
With MRI technology, it is now possible to identify stroke patients who have good collateral flow and substantial volumes of salvageable tissue many hours after symptom onset. In contrast, some patients develop large regions of irreversible injury rapidly. These patients, who are often said to have a “malignant profile,” remain difficult to treat (Figure 1). Data from the DEFUSE [Lansberg MG et al. Stroke 2007] and EPITHET studies found that if these patients were treated with tPA in the 3- to 6-hour treatment window, they had high rates of poor outcome (89% for tPA vs 33% for placebo; p<0.05). The most likely mechanism for this appears to be the high rate of hemorrhage following reperfusion observed in this patient population [Mylnash et al. Stroke 2011. In press].
Time is Brain.
Reproduced with permission from G. Albers, MD.
Dr. Albers briefly discussed the issue of mechanical recanalization and terminology misperceptions. He noted the term “FDA approval” is never appropriate to describe a device that is legally marketed under a 501(k). The correct term is “cleared for marketing” or “cleared by FDA.” Neurothrombectomy devices that are currently on the market in the United States have been cleared via the 501(k) pathway. Once these devices are cleared by the FDA, clinical research is required to clarify which patients benefit. The advances in stroke imaging that Dr. Albers highlighted can facilitate this goal, as evidenced by preliminary observations that large-vessel recanalization must result in reperfusion of salvageable tissue for substantial clinical benefits to occur.
“We now have the technology to perform studies that will change the paradigm,” Dr. Albers said. “Stroke treatment of the future should be about improving outcomes by rescuing salvageable tissue and treating the individual, not the time window.”
The editors would like to thank the many members of the 2011 International Stroke Conference presenting faculty who generously gave their time to ensure the accuracy and quality of the articles in this publication.
- © 2011 MD Conference Express