Summary
This article discusses the Carotid Revascularization Endarterectomy versus Stenting Trial [CREST; NCT00004732], which was a prospective, multicenter, randomized, controlled trial that compared carotid endarterectomy and carotid angioplasty and stenting in participants with symptomatic and asymptomatic stenosis.
- Interventional Techniques & Devices
- Valvular Disease
- Interventional Radiology
The Carotid Revascularization Endarterectomy versus Stenting Trial (CREST; NCT00004732) was a prospective, multicenter, randomized, controlled trial that compared carotid endarterectomy (CEA) and carotid angioplasty and stenting (CAS) in participants with symptomatic and asymptomatic stenosis. The primary endpoints were the periprocedural composite incidence of stroke, myocardial infarction (MI), and death, and the incidence of ipsilateral stroke up to 4 years postprocedure. There was no significant difference (p=0.38) in periprocedural outcome for the composite endpoint (for all patients, 5.2% in the CAS vs 4.5% in the CEA group [HR, 1.18; 95% CI, 0.82 to 1.69]), nor was there a significant difference (p=0.51) in the postprocedural outcome (for all patients, 7.2% in the CAS vs 6.8% in the CEA group [HR, 1.11; 95% CI, 0.81 to 1.51]) [Brott TG et al. New Engl J Med 2010].
The absence of significant outcome differences between CAS and CEA has been widely reported and the subject of much debate. In a session that discussed carotid angioplasty and endarterectomy, Robert Harbaugh, MD, Penn State University, Hershey, Pennsylvania, examined the CREST data in terms of stroke and MI incidence only. He reported that the rate of periprocedural stroke in all patients was 4.1% in the CAS group versus 2.3% in the CEA group (HR, 1.79; 95% CI, 1.14 to 2.82; p=0.01). The rate of MI was 1.1% in the CAS group versus 2.3% in the CEA group (HR, 0.50; 95% CI, 0.26 to 0.94; p=0.03). CEA is significantly more favorable regarding the stroke outcome, and CAS is significantly more favorable regarding MI outcome; however, when these values are combined in a composite endpoint, as was done in CREST, the values cancel each other out. A significant difference was also noted for all patients regarding the stroke and death rate to 4 years (6.4% for CAS versus 4.7% for CEA [HR, 1.50; 95% CI, 1.05 to 2.15; p=0.03]). When Dr. Harbaugh performed an analysis that was similar to what was done in CREST using data for 1500 CEAs (1195 symptomatic patients) from his own practice, he noted similar differences between the rates of ipsilateral stroke and MI.
In CREST, the lower incidence of stroke in the CEA group was associated with better quality of life outcome (SF-36) compared with the CAS group. Critics of CEA argue that CAS is less invasive than CEA; however, Dr. Harbaugh contends that when “minimally invasive” is defined as a procedure, which achieves the desired goal with the least disruption of the patient's homeostatic mechanisms, CEA is just as minimally invasive as CAS. CEA is a short procedure with a superficial surgical target and minimal blood loss that produces minimal distal embolization and, when performed with regional anesthesia, has a lower clinically significant complication rate than CAS.
Dr. Harbaugh concluded, “Based on the data from CREST and other recent trials, there is a growing body of evidence that CEA is superior to CAS for most patients with carotid artery disease. CAS should be reserved for those patients who are not good candidates for CEA.”
A different perspective was presented by Adnan H. Siddiqui, MD, PhD, State University of New York, Buffalo, New York, who presented data that showed no difference between CEA and CAS outcomes, contending that the two procedures are complementary strategies. Dr. Siddiqui reminded the group that the outcomes of CAS trials have improved significantly since the initiation of the CREST trial, whether one looks at a composite of death, stroke, and MI events (8.3% in 2000 compared with 2.3% in 2008) or a composite endpoint that includes only death and stroke rates (from 2.9% in 2000 to 0.6% in 2008). He noted that these decreases are due in large part to the increased experience of clinicians, better patient selection in the trials, and a wider spectrum of CAS technology.
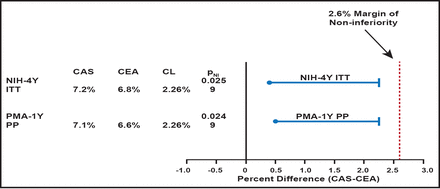
Moving to a direct discussion of the CREST data, Dr. Siddiqui pointed out that the data from CREST revealed a low event rate for both CAS and CEA for the composite (death, stroke, or MI to 30 days) endpoint and an acceptable benefit-risk profile. He also noted that in the premarketing and NIH analyses, CAS was statistically (p<0.05 for symptomatic [n=1219] and asymptomatic [n=1088] subgroups) noninferior to CEA. In the per-protocol 1-year premarket approval (PMA) analysis, CAS was noninferior to CEA (PNI =0.024; Figure 1) and consistent with the 4-year intent-to-treat (ITT) NIH analyses (PNI =0.025).
PMA and NIH Analyses.
Reproduced with permission from A. Siddiqui, MD.
Minor strokes occurred significantly (p=0.0088) more often with CAS (3.2%) compared with CEA (1.5%), while significantly (p=0.0387) more MIs occurred with CEA (3.4%) than CAS (2.0%). Importantly, there is no association between minor stroke and long-term mortality. There were statistically significantly (p=0.0001) fewer access-site complications in the CAS arm (1.1%) than the CEA arm (3.7%). Composite endpoint was higher for octogenarians for CAS and CEA. Freedom from clinically driven target lesion revascularization at 12 months by Kaplan-Meier survival analysis was similar (98.8% in the CEA arm and 99.0% in the CAS arm). The data show that 5.3% of CEA subjects had cranial nerve injury that remained unresolved in 3.6% of subjects at 1 month and in 2.1% of subjects at 6 months postprocedure. No subjects who received CAS reported cranial nerve injury. The 4-year long-term composite endpoint event rates (death, stroke, and MI plus ipsilateral stroke) between 31 days and 4 years were 8.8% in the CAS arm and 8.2% in the CEA arm with a hazard ratio (HR) of 1.08. Freedom from all-cause mortality to 4 years was 88.2% for CEA and 87.9% for CAS (HR, 1.19; 95% CI, 0.90 to 1.58; p=0.23).
In addition to being noninferior to CEA, CAS is associated with lower MI rates and long-term effectiveness to 4 years; it is considered less invasive, with fewer access-site complications; and has a lack of cranial nerve injury. However, there are higher rates of minor stroke at 30 days compared with CEA but similar residual neurological deficits at 6 months.
Overall, Dr. Siddiqui noted both CAS and CEA procedures have low event rates, which are lower than historical rates and within the American Heart Association guidelines for 30-day event rates. At experienced centers, both CEA and CAS appear to have low periprocedural complications and excellent longer-term results. Both treatments are viable options for standard-risk patients.
- © 2011 MD Conference Express