Summary
The Safety and Efficacy of NeuroFlo™ (CoAxia) Technology in Ischemic Stroke [SENTIS; NCT00119717] study is a multinational, randomized trial that evaluated partial abdominal aortic occlusion using the NeuroFlo™ catheter as a treatment for acute ischemic stroke.
- Interventional Techniques & Devices
- Ischemia Clinical Trials
The Safety and Efficacy of NeuroFlo™ (CoAxia) Technology in Ischemic Stroke (SENTIS; NCT00119717) study is a multinational, randomized trial that evaluated partial abdominal aortic occlusion using the NeuroFlo™ catheter as a treatment for acute ischemic stroke. Results were presented by Ashfaq Shuaib, MD, University of Alberta, Edmonton, Canada, and William P. Dillon, MD, University of California, San Francisco, California.
Treatment with NeuroFlo™ involves femoral access and placement of the catheter in the descending aorta, followed by sequential inflation of two independently controlled balloons in the infra- and suprarenal positions, each to 70% luminal occlusion, for 45 minutes. Preclinical studies in swine have demonstrated a 35% to 50% increase in cerebral blood flow with this technique [Hammer M et al. Cerebrovasc Dis 2009], and studies in small animals have shown a reduction in infarct size [Noor R et al. J Neuroimaging 2009]. Safety and indications of clinical benefit have been shown in human feasibility trials in ischemic stroke [Liebeskind D. Curr Cardiol Rep 2008]. The hypothesis is that partial occlusion of the abdominal aorta will augment cerebral blood flow via collateral perfusion, thus limiting stroke progression and improving outcomes.
The SENTIS trial included subjects (n=515) with a clinical diagnosis of stroke, a time from symptom onset of 0 to 14 hours, and a baseline NIH Stroke Scale (NIHSS) score of 5–18 and those who were ineligible for IV-tPA or thrombectomy. All subjects had baseline CT or MRI imaging to exclude hemorrhage and 24-hour scans postenrollment, when possible. Eligible subjects were randomly assigned to standard medical management or to NeuroFlo™ treatment plus the same medical management.
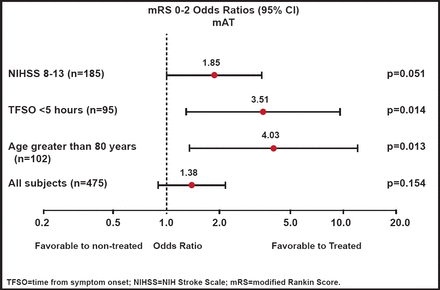
There was no significant difference in the overall population between the treatments in either the primary global efficacy score (OR, 1.17; p=0.407) or secondary endpoint of improvement in dichotomized modified Rankin Score (mRS) 0–2 (OR, 1.34; p=0.202), as measured in the predefined modified intent-to-treat population. Findings were similar when measured in the as-treated population, with a mRS 0–2 (OR, 1.38; p=0.154; Figure 1). Within the Rankin distribution, use of the NeuroFlo™ was associated with a 9% increase in subjects with a mRS score of 0–2 and a 30% decrease in those with a score of 5–6. A post hoc analysis revealed that subjects with NIHSS scores of 8–13 were more likely to benefit from NeuroFlo™ treatment (OR, 1.85; p=0.051) and that there was a significant (p=0.014) benefit associated with early treatment (<5 hours). Older age also influenced the benefit, particularly for subjects >80 years of age (OR, 4.03; p=0.013).
mRS 0–2 Outcomes (As-Treated Population).
Reproduced with permission from W. Dillon, MD.
In addition to the baseline and 24-hour required imaging, centers were encouraged to perform baseline and post-treatment perfusion imaging (MR or CT) and angiograms. For the imaging analysis, 280 baseline CT or MR perfusion studies and 196 follow-up CT or MR perfusion studies were available for analysis. From these two groups, 148 with paired pre- and posttherapy CT or MRI perfusion sets were available for review. A significant perfusion change was defined as: volume change >10 cc and percentage change >20% for relative mean transit time (rMTT)/mismatch, or volume change >5 cc and percentage change >20% for core infarct.
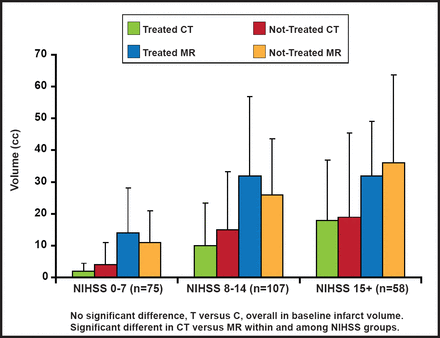
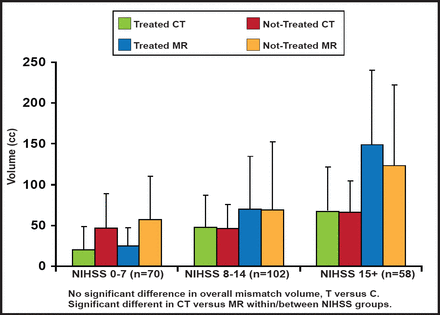
At baseline, there was no overall difference between treated and control groups with respect to core and mismatch volumes, but there was high variability in the mean. The core infarct and mismatch volumes were significantly larger on MRI versus CT across all NIHSS groups (Figures 2A and 2B). More proximal occlusions at baseline correlated with larger core infarcts and higher NIHSS scores. There were no significant differences between groups in the mean/median baseline CT or MR parameters (CBV, CBF, rMTT), measured on perfusion imaging. In terms of outcomes, however, those with MR mismatches at baseline showed improved responses to treatment, based on a 90-day dichotomized mRS 0–2 measure (OR, 15.75; 95% CI, 1.11 to 222.6; p=0.041); however, there was a large confidence interval.
Baseline Imaging Characteristics: Core Infarct Volume.
Reproduced with permission from W. Dillon, MD.
Baseline Perfusion Characteristics: Mismatch Volume.
Reproduced with permission from W. Dillon, MD.
- © 2011 MD Conference Express