Summary
This article discusses the results of the Apixaban Versus Acetylsalicylic Acid to Prevent Strokes [AVERROES; NCT00496769], which revealed clear evidence of a clinically important reduction in stroke and systemic embolism with apixaban over aspirin in patients with atrial fibrillation who were intolerant or otherwise considered unsuitable for vitamin K antagonist therapy.
- Cerebrovascular DiseaseArrhythmias Clinical Trials
- Cerebrovascular Disease
Hans-Christoph Diener, MD, University Hospital, Essen, Germany, presented the results of the Apixaban Versus Acetylsalicylic Acid to Prevent Strokes (AVERROES; NCT00496769) Study on behalf of the steering committee and the investigators. The study revealed clear evidence of a clinically important reduction in stroke and systemic embolism (SE) with apixaban over aspirin in patients with atrial fibrillation (AF) who were intolerant or otherwise considered unsuitable for vitamin K antagonist (VKA) therapy.
Patients with AF have an increased risk of stroke, and although VKA therapy is effective in reducing stroke, it is complex to manage and is associated with an increased risk of hemorrhage. For patients who are unsuitable for VKA therapy, the only alternative treatment is aspirin, which provides insufficient protection from stroke in high-risk patients (RR, 22%). Apixaban is an investigational oral anticoagulant that selectively inhibits factor Xa. It is efficacious as prophylaxis for venous thromboembolism and has a favorable risk-benefit ratio compared with low-molecular-weight heparin.
The purpose of the AVERROES Study was to evaluate apixaban for the prevention of stroke or SE patients with AF who are at risk for stroke and unsuitable for VKA therapy. AVERROES was a double-blind, randomized, active comparator trial that compared apixaban with aspirin. Patients with documented AF and at least one risk factor for stroke who were also unsuitable for VKA therapy were randomly assigned to receive either apixaban 5 mg bid (n=2808) or aspirin 81–324 mg per day (n=2791). The primary study outcome was stroke or a systemic embolic event (SEE).
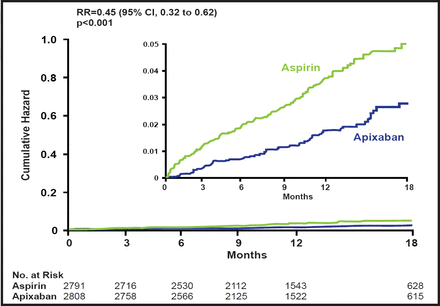
Subjects had a mean age of 70 years, and 59% were men. Approximately 75% of subjects were on aspirin therapy at baseline, and 40% had been on VKA therapy previously but had discontinued its use; 60% were considered unsuitable for VKA therapy. AVERROES was stopped early, based on overwhelming evidence of efficacy against stroke or SE, together with an excellent safety profile. The relative risk reduction in favor of apixaban was 0.45 (95% CI, 0.32 to 0.62; p<0.001; Figure 1).
Stroke or SEE.
Copyright © 2011 Massachusetts Medical Society. All rights reserved.
When the primary outcome events were assessed separately, there were 49 strokes with apixaban versus 105 with aspirin (RR, 0.46; 95% CI, 0.33 to 0.65; p<0.001) and 2 SEEs with apixaban versus 13 with aspirin (RR, 0.15; 95% CI, 0.03 to 0.68; p=0.01). There was no difference in the secondary outcomes of myocardial infarction, vascular death, or mortality. Hospitalizations for cardiovascular events were significantly lower with apixaban (367 vs 455; p<0.001).
Apixaban was well tolerated, with no evidence of liver toxicity. There was a small increase in bleeding with apixaban compared with aspirin, but the difference was not significant (RR, 1.13; 95% CI, 0.74 to 1.75; p=0.57). Adverse events were similar in both groups, with the exception of nervous system disorders, which were significantly more common in the aspirin group (6.6%) compared with the apixaban group (3% of patients; p<0.001) [Connolly SJ et al. N Engl J Med 2011].
- © 2011 MD Conference Express