Summary
The Scandinavian Candesartan Acute Stroke Trial [SCAST; NCT00120003] showed that routine blood pressure (BP)-lowering treatment in patients with acute stroke and elevated BP had no benefit and could have a potentially harmful effect.
- Cerebrovascular Disease
- Hypertensive Disease Clinical Trials
Else C. Sandset, MD, Oslo University Hospital, Ullevål, Oslo, Norway, presented the results of the Scandinavian Candesartan Acute Stroke Trial (SCAST; NCT00120003), which showed that routine blood pressure (BP)-lowering treatment in patients with acute stroke and elevated BP had no benefit and could have a potentially harmful effect.
SCAST was a multicenter, double-blind, randomized, placebo-controlled trial of candesartan in patients (n=2029) with acute stroke and elevated BP. The objective was to assess whether BP-lowering with candesartan was beneficial in the setting of acute stroke. Adult patients with a clinical diagnosis of stroke (ischemic or hemorrhagic) and systolic BP ≥140 mm Hg for whom treatment was possible within 30 hours of the onset of symptoms were eligible for inclusion.
Subjects were randomly assigned to candesartan (n=1017) or placebo (n=1012) for 7 days. The dose of candesartan increased from 4 mg on Day 1 to 16 mg on Days 3 to 7. Treatment during the follow-up period was at the discretion of the clinician. BP was monitored daily during the treatment phase and during the follow-up visits at 1, 3, and 6 months. The coprimary endpoints were: a composite vascular endpoint, comprising vascular death, myocardial infarction (MI), and stroke during the first 6 months; and functional outcome at 6 months, as measured by modified Rankin Scale. To adjust for the use of two coprimary effect variables, a p-value of 0.025 or 0.05 was required for one or both variables, respectively, for the results to be considered significant.
Subjects had a mean age of 71 years and a mean BP at baseline of 171/90 mm Hg. Mean duration of symptoms prior to inclusion was 18 hours. Ischemic stroke was present in 85% of subjects; hemorrhagic stroke was present in 14%. Over the 7-day treatment period, BP was significantly lower among patients who received candesartan, with a mean BP of 147/82 mm Hg, versus 152/84 mm Hg in the placebo group (p<0.001). BP reduction was observed as early as Day 2.
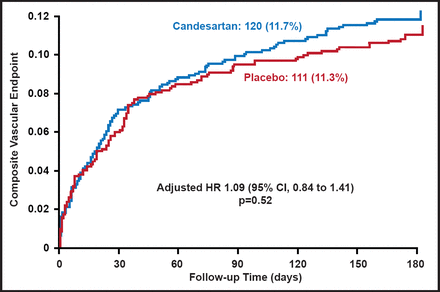
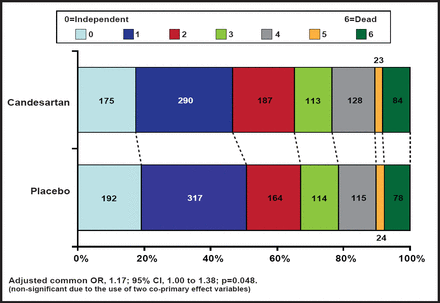
There was no difference in the composite vascular endpoint of vascular death, MI, and stroke (Figure 1). There was also no difference in the coprimary endpoint of functional outcome, although there was a consistent shift in all categories of the modified Rankin Scale in favor of placebo (adjusted common OR, 1.17; 95% CI, 1.00 to 1.38; p=0.048; Figure 2). Across all secondary endpoints, there was a nonsignificant increased risk in the candesartan-treated group. For stroke progression (6% of subjects in the candesartan group and 4% of placebo subjects), the relative risk was 1.47 (95% CI, 1.01 to 2.13; p=0.04).
Composite Vascular Endpoint: Vascular Death, MI, or Stroke.
Reproduced with permission from E. Sandset, MD.
Functional Outcome (mRS).
Reproduced with permission from E. Sandset, MD.
There was no evidence of a differential effect in any of the subgroup analyses (eg, stroke subtype, systolic BP, duration of symptoms, history of hypertension), with the exception of a trend that favored candesartan in subjects with a symptom duration <6 hours for the composite vascular endpoint.
The results of SCAST were confirmed by a meta-analysis of clinical trials of BP-lowering in acute stroke, comprising more than 100 subjects.
- © 2011 MD Conference Express