Summary
This article discusses the scientific and clinical targets for acute intervention including immunomodulation, the benefits of direct thrombolytic plasmin, the use of activated protein C for chronic neurodegenerative disorders, as well as strategies to modify the endothelial hemostatic-thrombotic balance to treat stroke.
- inflammatory diseases
- systemic atrophies
- neurological autoimmune diseases
- cerebrovascular disease
Immunomodulation
Inflammatory and immune mechanisms are a significant deleterious part of the stroke process. However, through immunomodulation, it is possible to attenuate brain damage following a stroke. This can be achieved through a process called mucosal tolerance, which involves the repetitive introduction of low-dose antigens either nasally or orally to tolerize regulatory T cells (Tregs). When next presented with the antigen to which they have been primed, Tregs will suppress inflammation (even if it is not caused by the antigen to which they have been tolerized) and provide local immunosuppression, a process termed “bystander suppression.”
John Hallenbeck, MD, National Institutes of Health, Bethesda, MD, described current research with E-selectin, a cell adhesion molecule that is only expressed on endothelial cells when they are becoming activated. The local release of the cytokines IL-1 and TNF by the inflamed cells induces the expression of E-selectin on the endothelial cells of nearby blood vessels. Dr. Hallenbeck's previous research showed that E-selectin prevented spontaneous ischemic and hemorrhagic strokes in spontaneously hypertensive, genetically stroke-prone rats compared with controls [Takeda H et al. Stroke 2002], and further, mucosal tolerance to E-selectin significantly reduced infarct volume following permanent middle cerebral artery occlusion in these animals [Chen Y et al. Proc Natl Acad Sci USA 2003].
His current research in a vascular cognitive impairment model (common carotid occlusion) showed that E-selectin-tolerized animals have significantly less learning impairment compared with controls. Dr. Hallenbeck's research has also shown that mucosal tolerance to E-selectin increases Tregs in peri-infarct regions after stroke, promotes the survival of migrating neuroblasts and newly generated neurons, and can suppress a Th-17 (T cells that produce IL-17)-driven autoimmune disease. With this knowledge, Dr. Hallenbeck's team hopes to begin safety trials that test immunomodulation as a tool for the prevention of stroke.
Plasmin
There are two types of thrombolytics, indirect and direct. Indirect (tPA, SK, UK, reteplase, etc) convert plasminogen to plasmin by way of an activator. Direct (plasmin, mutant derivatives, and snake venom extracts such as alfimeprase) degrade fibrin directly without the need for a precursor. Although plasminogen activators are effective for dissolving thrombi, their use is associated with an increased risk of bleeding, including intracranial hemorrhage (about 1% of patients), that can complicate regional as well as systemic therapy.
Victor J. Marder, MD, David Geffen School of Medicine, Los Angeles, CA, discussed the benefits of the direct thrombolytic plasmin. Plasmin is inhibited by antiplasmin in the general circulation; thus, it is never administered systematically because it will be neutralized and never reach the thrombus. However, administered locally, it dissolves the thrombus with no risk of ancillary bleeding, while providing a significant safety margin.
In a middle cerebral artery thrombo-occlusion model in rabbits, thrombolysis/reperfusion was achieved in 3 of 3 rabbits that were successfully administered plasmin by interarterial infusion [Jahan R et al. Stroke 2001]. In a phase 1 clinical trial, plasmin produced effective thrombolysis (>75%) at 24 mg with no major bleeding [Shlansky-Goldberg et al. J Thromb Haemost in press]. Plasmin is undergoing further testing in the Plasmin Revascularization for the Ischemic Lower Extremity (PRIORITY) trial.
Dr. Marder believes that plasmin is the ideal agent for catheter-delivered thrombolysis in that it causes less bleeding than tPA, is likely superior to tPA for lysis of plasminogen-poor thrombi, and has a highly favorable benefit-to-risk ratio.
Activated Protein C (APC)
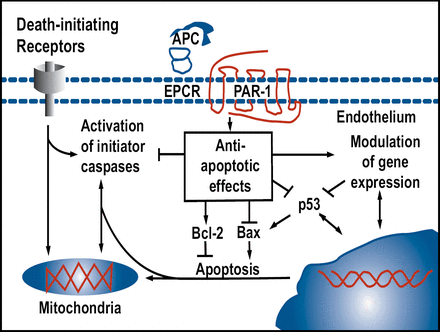
Berislav Zlokovic, MD, University of Rochester Medical Center, Rochester, NY, discussed the need for new therapeutic approaches that are directed at the blood-brain barrier and other non-neuronal cells for chronic neurodegenerative disorders. Activated protein C (APC) is an antithrombotic, anti-inflammatory, and profibrinolytic agent that first drew attention as a successful treatment for sepsis in the PROWESS study [Bernard GR et al. NEJM 2001]. APC reduced the relative risk of death of patients with severe sepsis by 19.4%. In mouse cortical neurons, APC blocks apoptosis that is induced by N-methyl-D-aspartate (NMDA) and staurosporine, demonstrating direct protection of neurons and suggesting that it might be a useful treatment for amyotrophic lateral sclerosis (ALS), Alzheimer disease, and ischemic stroke [Guo H et al. Neuron 2004]. APC also activates anti-apoptotic pathways in brain endothelium that is affected by ischemia or tPA in cultured brain endothelium and in vivo (Figure 1). APC is currently being investigated in patients with acute ischemic stroke in the Activated Protein C in Acute Stroke Trial.
Activated Protein C (APC): Modeling Stroke.
A more recent study [Cheng T et al. Nature Medicine 2003] showed that APC inhibits a prohemorrhagic, tPA-induced, NF-kappaB-dependent matrix metalloproteinase-9 pathway in ischemic brain endothelium in vivo and in vitro by acting through protease-activated receptor 1. This suggests that APC may improve thrombolytic therapy for stroke in part by reducing tPA-mediated hemorrhage.
Dr. Zlokovic is planning phase 2/3 clinical trials that look at the angiogenesis, neurogenesis, neuronal re-wiring, vascular remodeling, and metabolic coupling properties of APC.
Modulating Endothelial Cell Anticoagulant Properties
Mark Fisher, MD, University of California, Irvine, CA, discussed strategies to modify the endothelial hemostatic-thrombotic balance as a way to treat stroke. Endothelial cells express a variety of pro- and antithrombotic properties. The antithrombotic properties include the production of prostacyclin, thrombomodulin, nitric oxide, and tPA. Prothrombotic factors include tissue factor, Von Willebrand factor, and plasminogen activator (PAI-1).
Referencing previous research, Dr. Fisher pointed out that thrombomodulin can transform thrombin's usual procoagulant effects into anticoagulant function via the thrombin-thrombomodulin complex, which activates protein C. Thrombomodulin is ubiquitous in endothelial cells throughout the systemic microcirculation but is reduced at the blood-brain barrier; areas that are predisposed to lacunar infarction have a particularly low abundance of thrombomodulin [Ishii H et al. Blood 1986; Wong VL et al. Brain Res 1991]. Thrombomodulin is easily demonstrable in microvasculature of brain tumors, in contrast to microvessels of normal brain [Isaka T et al. Acta Neuropathol 1994], and normal brain capillary endothelial cells rarely express tPA [Levin and Del Zoppo. 1994]. This underexpression of anticoagulant factors by brain microvascular endothelial cells, coupled with abundant expression of tissue factor by blood-brain barrier astrocytes, may predispose to ischemia in prothrombotic or inflammatory conditions.
Dr. Fisher offered 3 strategies to modify endothelial anticoagulant function that included using statins, using phosphodiesterase inhibitors, and inhibiting the renin-angiotensin system. Manipulating the prothrombotic properties of the brain may offer protection against infarction.
He concluded by saying that the brain contains procoagulant properties based in the microvasculature, which presents multiple potential therapeutic targets to alter endothelial anticoagulant function as stroke intervention.
- © 2008 MD Conference Express