Summary
Intracerebral hemorrhage (ICH) mortality estimates range from 37% (STICH trial) to 56% (SCHIPA trial). The Minimally Invasive Surgery plus t-PA for Intracerebral Hemorrhage Evacuation [MISTIE] trial was a phase 2 study sponsored by the National Institute of Neurological Disease and Stroke. The objective of the study was to determine whether reduction of clot size in ICH leads to lower mortality and better outcomes for patients.
- interventional techniques & devices
- ischemia clinical trials
Intracerebral hemorrhage (ICH) mortality estimates range from 37% (STICH trial) to 56% (SCHIPA trial). The Minimally Invasive Surgery plus t-PA for Intracerebral Hemorrhage Evacuation (MISTIE) trial was a phase 2 study sponsored by the National Institute of Neurological Disease and Stroke. The objective of the study was to determine whether reduction of clot size in ICH leads to lower mortality and better outcomes for patients. Daniel F. Hanley, MD, Johns Hopkins University, Baltimore, MD, presented recent findings from this study on behalf of Mario Zuccarello, MD, University of Cincinnati, Cincinnati, OH, and the MISTIE Investigators.
The hypothesis of this ongoing trial is that clot reduction that uses minimally invasive surgery (MIS) combined with recombinant tissue plasminogen activator (rt-PA) is safe and that clot size reduction would be better than in medically treated patients. In order to enter the trial, patients had to have met the following criteria: be between the ages of 18–80 years, have a Glasgow Coma Scale score ≤14 or an NIH Stroke Scale ≥6, an ICH ≥25 cc, a stable clot at a second CT scan 6 hours later, systolic blood pressure of <200 mm Hg or MAP <130 mm Hg over 6 hours, and historical Rankin of 0 or 1. Patients with brain tumors, infratentorial ICH, intraventricular hemorrhage with external ventricular drainage management, irreversibly impaired brain stem function, major medical diseases, or who were pregnant were excluded. Patients were stratified by ICH size (25–50 mL and >50 mL). The study design used a sequential tier approach, the first tier being 0.3 mg of rt-PA. After giving consent, patients underwent MIS. The cannula and catheter were placed at 2/3 of the clot long axis and in the middle 1/3 of clot width. The clot was then aspirated until resistance occurred. The placement of the catheter was confirmed by CT scan, and 0.3 mg rt-PA was administered every 8 hours until the clot was reduced to 15 cc or 80% of the original clot size, whichever occurred first.
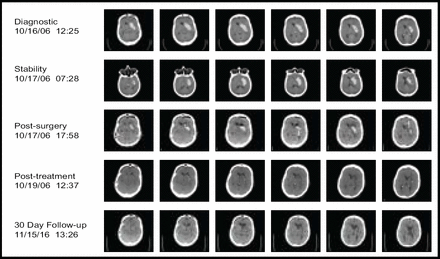
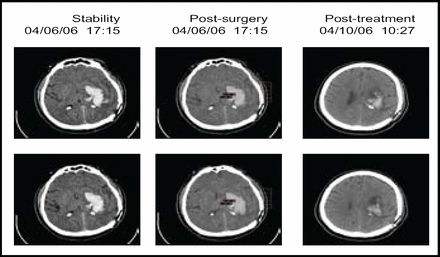
A total of 20 subjects received treatment in this tier, with 73% achieving the clot removal goal. Figure 1 contains sequential scans from a patient in the trial. The procedure had an acceptable safety profile (mortality 5%, no cerebral infections, and 10% symptomatic bleeding). Furthermore, 20% of the subjects needed only aspiration to reach the endpoint. The results indicated that catheter placement is critical, as clot resolution was not as effective if the catheter was not placed in an optimal position (Figure 2). The next steps in the MISTIE trial are to evaluate the risks and benefits of a 1.0-mg rt-PA dose, improve on the clot lysis rate and efficiency, and gather additional safety data. More information regarding this study is available at www.mistietrial.com.
MIS + rt-PA: Thumbnail Experience.
Poor Placement (Subject B).
- © 2008 MD Conference Express