Summary
The period of doubt concerning the safety of drug-eluting stents (DES) began in 2004 with the publication of the first report of late stent thrombosis [McFadden et al. Lancet 2004]. Despite what appeared to be conflicting reports from the SCAAR and GRACE registry studies, it is apparent that much progress has been made in understanding late stent thrombosis. This article reviews the results of several recently published meta-analyses that have evaluated the safety of DES vs bare-metal stents.
- thrombotic disorders
- interventional techniques & devices
The period of doubt concerning the safety of drug-eluting stents (DES) began in 2004 with the publication of the first report of late stent thrombosis [McFadden et al. Lancet 2004]. The debate reached its peak at the World Congress of Cardiology in Barcelona in 2006 and was continued at this year's Annual Meeting of the European Society of Cardiology in Vienna.
Despite what appeared to be conflicting reports from the SCAAR and GRACE registry studies (see the Late Breaking Clinical Trial section of this issue of MDCE), it is apparent that much progress has been made in understanding late stent thrombosis. Thierry Lefèvre, MD, Institut Hospitalier Jacques Cartier, Massy, France, reviewed the results of several recently published meta-analyses that have evaluated the safety of DES vs bare-metal stents (BMS). The results of these studies, which are based on “on-label” individual patient data, indicate that although there is a trend toward increased mortality with DES, the differences are not significant vs BMS.
Using the standard ARC (Academic Research Consortium) definition for stent thrombosis, Mauri and colleagues analyzed pooled 4-year follow-up data for 4,545 patients from 8 randomized trials in which patients were treated with sirolimus-eluting stents (SES; n=878), paclitaxel-eluting stents (PES; n=1,400), or with BMS (n=2,267). The study results showed no difference in the incidence of stent thrombosis between either DES and BMS (SES 1.5% vs 1.7% BMS; p=0.70; 95% CI 1.5, 1.0: and PES 1.8% vs BMS 1.4% p=0.52; 95% CI 0.7, 1.4) [Mauri L et al. N Engl J Med 2007].
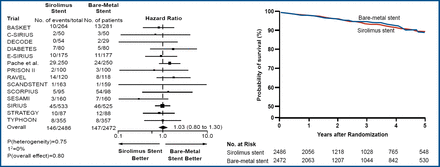
In a study designed to evaluate the long-term effects of treatment with SES vs BMS, Kastrati and colleagues analyzed individual data from 4,958 patients enrolled in 14 randomized trials (mean follow-up 12.1–58.9 months). The primary endpoint was death from any cause. Other outcomes included stent thrombosis, the composite endpoint of death or myocardial infarction (MI), and the composite of death, MI, or need for repeat intervention.
The study results indicated no difference in either overall mortality (Figure 1) or the composite endpoint of death or MI (HR 0.97; 95% CI 0.81, 1.16) between patients with SES and those with BMS during a 5-year period (Figure 1).
Meta-Analysis of 5-Year Data from 14 Clinical Trials: Any Death.
Copyright © 2007 Massachusetts Medical Society. All rights reserved.
Although there was no significant increase in the overall rate of stent thrombosis in patients with SES, stent thrombosis was significantly more frequent (p=0.02) after the first year following the procedure and at a time that appeared to be associated with the discontinuation of antiplatelet therapy [Kastrati A et al. N Engl J Med 2007].
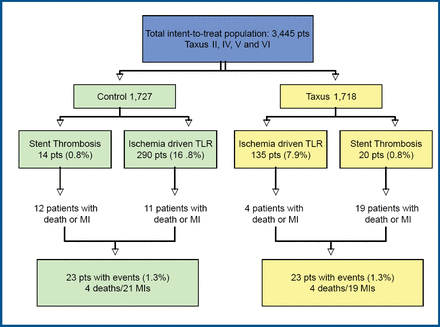
Although these, and other studies, have shown that DES (vs BMS) may increase late stent thrombosis, there has not been a corresponding increase in the rates of death and MI. Results of a study conducted by Stone and colleagues indicate that there is a marked reduction in restenosis with DES, which may counterbalance their potential excess risk from late stent thrombosis [Stone GW et al. Circulation 2007]. In that study, patient-level data from 3,445 patients from 4 prospective, double-blind trials (median follow-up 3.2 years) were analyzed to assess the occurrence of death or MI within 7 days of stent thrombosis or target lesion revascularization (TLR). Patients were randomly assigned to either PES or BMS. Stent thrombosis occurred in 14 BMS and 20 PES patients. Before 1 year, thrombosis occurred in 12 patients in each group. After that, stent thrombosis appeared in 2 BMS and 8 PES patients (p=0.06). There were 12 deaths or MIs in the BMS group and 19 in the PES group within 7 days of thrombosis (p=0.56). TLR was performed in 290 BMS and 135 PES (p<0.0001). There were 11 deaths or MIs in the BMS group and 4 in the PES group within 7 days of TLR (p=0.78; Figure 2). In total, 23 patients in both groups died or had an MI within 7 days of either stent thrombosis or TLR.
Impact of Thrombosis and Restenosis.
Other studies have investigated possible independent predictors of late stent thrombosis. In a study which analyzed data for 1,731 patients from the EVASTENT registry, Machecourt and colleagues found that interruption of antithrombotic treatment, previous stroke, renal failure, lower ejection fraction, calcified lesion, longer stents, and insulin-dependent diabetes were all independent predictors of stent thrombosis. In particular, they found that the 1-year stent thrombosis rate was 1.8 times higher in diabetic vs nondiabetic patients (3.2% vs 1.7%; p=0.03); diabetic patients with multiple-vessel disease experienced the highest rate of thrombosis while non-diabetic patients with single-vessel disease had the lowest (4.3% vs 0.8%; p<0.001) [Machecourt J et al. JACC 2007].
Previous studies have also shown an association between stent thrombosis after successful SES implantation and stent underexpansion and residual reference segment stenosis [Fujii K et al. J Am Col Cardiol 2005], geographical miss [Costa MA. ACC 2006], and duration of antiplatelet therapy [Chieffo A. TCT 2006].
According to Prof. Thierry, stent thrombosis is a multifactorial problem that is comprised of technical issues (underexpansion, overlapping, technique), individual patient profile (AMI, diabetes, renal failure), lesion characteristics (diameter, length, number of vessels), and stent construction (drug, design, material). Recognition of all of these issues can lead to improved patient safety.
A New Generation of DES
The goal in the use of bioabsorbable stents is to allow for natural healing, normal vasomotion, and late expansive modeling by eliminating the permanent metallic implant, to potentially shorten the duration of antiplatelet therapy by minimizing inflammation, and to facilitate repeat intervention. Additional benefits include the potential for non-invasive follow-up via MRI and CT scan, and increased drug-loading capacity that may permit continuous drug release strategies.
Patrick Serruys, MD, Thoraxcentre, Rotterdam, the Netherlands, presented the 6-month angiographic and intravascular ultrasound (IVUS) results of the first-in-man use of the bioabsorbable everolimus eluting coronary stent system (BVS). The ABSORB study assessed the safety and performance of the BVS in the treatment of patients with a single de novo lesion (3.0 mm in diameter). Thirty patients (58% men) were enrolled at 4 clinical sites. Quantitative coronary angiography (QCA) and IVUS were performed at 6 months. QCA results are available for 26 patients; IVUS results are available for 24 patients. QCA showed a median in-stent loss of 0.39mm (mean 0.44mm; 95% CI 0.30, 0.58). Three patients (11.5%) experienced binary restenosis. The stenoses were not severe (50–55%). All 3 patients were asymptomatic; none underwent TLR. IVUS results (Table 1) showed no vascular remodeling (0.3%, p=NS), but an 11.7% (p<0.001) reduction in stent area suggesting late stent recoil. Overall the in-stent volume obstruction was 5.5±8.5%. In 11 patients there was no detectable neointimal hyperplasia; some degree of neointimal hyperplasia was detected in 13 patients. In 13 patients with both late recoil and neointimal hyperplasia, the in-stent volume obstruction was 10.2±9.2%. The rate of major adverse cardiac events was low (3.3%).
IVUS Results (24 Patients).
“The encouraging results from the first 30 patients of ABSORB suggest that drug-eluting bioabsorbable stent technologies may be a promising future therapy option for physicians treating patients with heart disease,” said Prof. Serruys, co-principal investigator of the study. “A drug-eluting stent that would eventually disappear after restoring blood flow is an exciting concept that we look forward to further exploring.”
- © 2007 MD Conference Express