Summary
Cardiogenic shock accounts for about one half of the in-hospital mortality after an acute myocardial infarction. Rapid diagnosis and mechanical reperfusion can improve survival. Mechanical support is an important tool to manage but profound shock or cardiovascular collapse. Extracorporeal life support and other technologies are promising but additional evidence is needed to determine their impact on mortality.
- cardiogenic shock
- acute myocardial infarction
- intra-aortic balloon pump
- SHOCK
- IABP-SHOCK II
- extracorporeal life support
- percutaneous coronary intervention
- impella
- TandemHeart
- extracorporeal membrane oxygenation
- cardiology & cardiovascular medicine clinical trials
- interventional techniques & devices
With an incidence between 5% and 10% and an in-hospital mortality rate of about 50%, cardiogenic shock (CS) is the leading cause of death among patients hospitalized for acute myocardial infarction (AMI) [Kolte D et al. J Am Heart Assoc. 2014; Hochman JS. Circulation. 2003]. Khaled M. Ziada, MD, Gill Heart Institute, University of Kentucky, Lexington, Kentucky, USA, discussed trends in the treatment of this dangerous condition.
According to Dr Ziada, the primary objectives for the management of CS are to support brain and kidney function; prevent or reverse acidosis; and reverse vasodilation, hypoxemia, and systemic hypoperfusion as quickly as possible. The use of mechanical hemodynamic (HD) support (eg, intra-aortic balloon pump [IABP]) is broadly accepted as a necessary intervention despite the paucity of randomized trial data. Registry data indicate that IABP improves the cardiac index and coronary perfusion while reducing afterload and left ventricular end-diastolic pressure. However, IABP is a temporary measure that does not decrease mortality.
The majority (86%) of subjects in both arms of the SHOCK trial [Hochman JS et al. JAMA. 2006] received IABP support. Although emergency revascularization did not significantly reduce overall mortality at 30 days, 6-month mortality was lower in the revascularization group versus the medical therapy group (50.3% vs 63.1%; P = .027). After 6 years, 62.4% of hospital survivors with CS who were treated with early revascularization were still alive, compared with 44.4% of those treated medically. Compared with initial medical stabilization, this represents a 13.2% absolute improvement and a 67% relative improvement in 6-year survival.
In the IABP-SHOCK II trial [Thiele H et al. N Engl J Med. 2012], 600 patients with AMI and CS were randomized to IABP plus medical therapy or medical therapy only. After 30 days, all-cause mortality was similar between 2 groups—41.3% versus 39.7% in the control and IABP groups, respectively (log-rank P = .92).
Mechanical devices such as the Impella, the TandemHeart, and extracorporeal life support (ECLS; also known as extracorporeal membrane oxygenation [ECMO]) have recently gained popularity as replacements for IABP. The Impella is a miniaturized pump motor that is placed into the left ventricle with inlet and outlet holes straddling the aortic valve. It is delivered via a standard catheterization procedure through the femoral artery (Impella 2.5 and Impella CP) or, in the case of the Impella 5.0, via femoral cutdown or through the axillary artery. The pump pulls blood from the left ventricle through an inlet area near the tip and expels blood from the catheter into the ascending aorta. Axial flow ranges from 2.5 to 5.0 L/min for the Impella 2.5 and 5.0; the Impella CP can deliver 3.5 to 4 L/min.
In the ISAR-SHOCK trial [Seyfarth M et al. J Am Coll Cardiol. 2008], the Impella 2.5 reduced lactate and provided superior HD support when compared with the IABP, although hemolysis was a problem. This study included only 26 patients, and there are no public data for the Impella 5.0 or CP. While there are no clinical data showing an improvement in survival, the US Impella registry for AMI noted that patients receiving the Impella device pre–percutaneous coronary intervention (PCI) had significantly better survival-to-discharge rates when compared with patients receiving the device post-PCI (65.1% vs 40.7%; P = .003) [O'Neill WW et al. J Interv Cardiol. 2014].
The TandemHeart is a percutaneous bypass device—left atrial to iliac artery—powered by an external centrifugal pump that provides up to 3.5 to 4 L/min of forward flow. To access the left atrium as well as the iliac artery, arterial and venous access must be obtained at the femoral vessels [Naidu SS. Circulation. 2011]. There have been no large-scale clinical trials with this device, but there is some evidence that it provides effective HD support superior to IABP (Table 1). The technically demanding insertion technique has been the limiting factor for a more widespread use.
TandemHeart Provides Superior Hemodynamic Results Relative to IABP
ECLS/ECMO is a percutaneous cardiopulmonary bypass system that provides complete support of cardiac output and respiratory function. Femoral or neck access and both arterial and venous access are possible. Although insertion is not time-consuming, a perfusionist is needed. To date, no randomized study data are available for ECLS/ECMO use in CS.
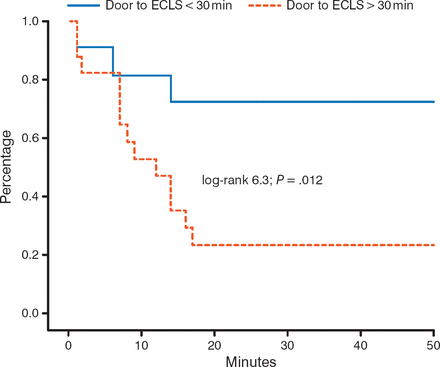
Observational data suggest that timing of implantation is critical. A door-to-ECLS implantation time of < 30 minutes, as compared to > 30 minutes, significantly (P = .012) improves 30-day outcomes in patients with out-of-hospital cardiac arrest (Figure 1) [Leick J et al. Clin Res Cardiol. 2013].
Impact of Time to ECLS Implantation on 30-Day Survival
ECLS, extracorporeal life support.
Adapted from Clinical Research in Cardiology, 102, 2013, 661–669. Door-to-implantation time of extracorporeal life support systems predicts mortality in patients with out-of-hospital cardiac arrest. Lieck J et al. Fig 1. © Springer-Verlag Berlin Heidelberg 2013. With kind permission from Springer Science and Business Media.
There are 2 strategies for ECLS use. The first focuses on acute stabilization, short-term transfer, and moratorium of decision. Duration of support is < 72 hours, with rapid deployment of nondurable technology. The second is an integrated program with a focus on recovery and using ECLS as a bridge to transplant. Duration of support is > 72 hours and requires extended infrastructure and durable technologies.
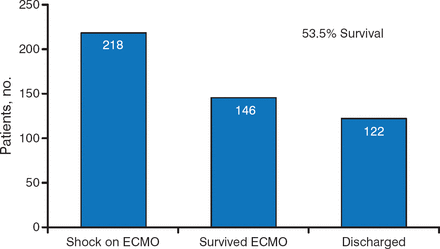
While data showing good outcomes with ECLS are limited, there is cause for optimism. In a recent study of the 218 patients who went into shock on ECMO, 146 survived, and 122 (53.5%) were successfully discharged (Figure 2) [Loforte A et al. Artif Organs. 2014]. While this observation is nonrandomized and with no comparison, the rates of survival appear encouraging when compared to the known natural history of CS.
Number of Patients Who Survived and Were Discharged Following ECLS
ECLS, extracorporeal life support; ECMO, extracorporeal membrane oxygenation.
Source: Loforte A et al. Artif Organs. 2014. Reproduced with permission from KM Ziada, MD.
Bleeding, arterial injury, hemolysis, thrombocytopenia, and transseptal puncture are some of the complications noted with the use of mechanical devices.
CS is highly fatal in patients with AMI and requires rapid diagnosis and mechanical reperfusion to improve survival. The primary objective is to save brain and kidney function. Mechanical support, a mainstay of HD maintenance, is an important tool for the management of profound shock or cardiovascular collapse. Timing of mechanical device implantation and patient selection are critical for success. Technologies such as ECLS are promising, but such devices can be technically challenging and have a learning curve. Additional data are required to determine if the various forms of mechanical support affect mortality.
- © 2015 SAGE Publications