Summary
Novel technologies for the percutaneous treatment of mitral regurgitation and stenosis are being developed and evaluated. Evidence with the Endovalve-Herrmann prosthesis for mitral valve replacement and the MitraClip System for leaflet repair were reviewed in this article.
- mitral regurgitation
- stenosis
- heart failure
- pulmonary hypertension
- cardiology & cardiovascular medicine clinical trials
- interventional techniques & devices
- valvular disease
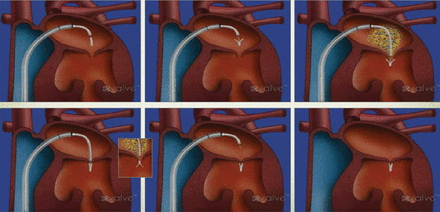
Ramesh Daggubati, MD, East Carolina Heart Institute, Greenville, North Carolina, USA, described some of the percutaneous technologies on the horizon for treating mitral regurgitation (MR) and stenosis. He began with the case of an 86-year-old man with a history of diabetes, hypertension, NYHA class III congestive heart failure, and pulmonary hypertension, who had undergone coronary artery bypass surgery in 1994 and received an implantable cardioverter defibrillator in 2007. The patient developed severe MR but was not considered a candidate for mitral valve (MV) surgery. Instead, he was treated in the catheterization laboratory using 2 MV leaflet clips (Figure 1). The patient was transferred to the general ward on day 1 and discharged 4 days later. At 9 months, the patient had only mild residual MR and there had been reductions in left ventricular (LV) volume. The patient's symptoms improved and he continues to do well 4 years after implantation of the mitral clip.
Deployment and Placement of Mitral Valve Leaflet Clip
Reproduced with permission from R Daggubati, MD.
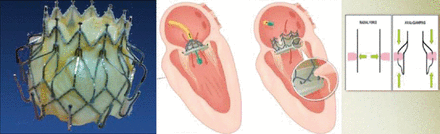
The Endovalve-Herrmann prosthesis is a novel device that is currently being developed for MV replacement. The implant consists of a foldable nitinol structure with specially designed grippers that are repositionable before release. A minithoracotomy is performed on the beating heart and the device is implanted from the left atrial side. Because of the difficulty of keeping the valve in place, another device is being developed that is delivered transseptally and locks onto the inferior and superior surfaces of the mitral annulus. This device has a self-expanding, bi-level nitinol frame and 2 sets of opposing anchors (Figure 2).
Mitral Valve Self-Expanding Replacement Device (CardiAQ)
Reproduced with permission from R Daggubati, MD.
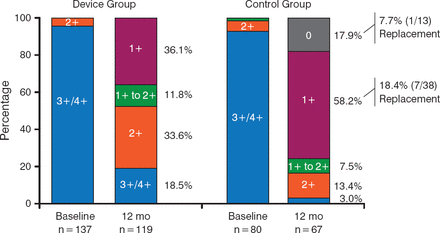
EVEREST II [NCT00209274] was the first pivotal US trial for leaflet repair with a clip device. In this study, 279 patients with moderate-to-severe MR (grade 3+ or 4+) were randomly assigned (2:1 ratio) to either percutaneous MV repair with the MitraClip System or to conventional surgical repair/replacement. At 12 months, grade 3+ or 4+ MR was 21% in the percutaneous-repair group and 20% in the surgery group (Figure 3). Both groups had improved LV size, NYHA functional class, and quality-of-life measures compared with baseline [Feldman T et al. N Engl J Med. 2011].
Reduction in Mitral Regurgitation After MitraClip Placement or Surgical Repair
Reproduced with permission from R Daggubati, MD.
The COAPT trial [NCT01626079] is currently enrolling participants and is randomizing high-risk patients with functional MR (≥ 3+)to treatment with either the MitraClip or standard medical care. This trial and its European counterpart, RESHAPE, are expected to evaluate whether there is a role for the MitraClip in patients with functional MR.
Dr Daggubati also presented a case in which a novel approach was undertaken in a 59-year-old woman with a history of repeated admissions for shortness of breath and 2 prior MV replacements. She was treated off-label with a 26-mm Edwards SAPIEN valve that was deployed into a degenerative bioprosthetic M V. After the procedure, the patient demonstrated no MR or perivalvular leaks, and her cardiac output was 5.2 L/min. Her symptoms improved and she was reclassified from NYHA class IV to NYHA class I.
Mitral stenosis can also be treated with percutaneous mitral valvuloplasty, although Dr Daggubati noted that some conditions are not amenable to percutaneous therapies (Table 1).
Contraindications for Percutaneous Mitral Valvuloplasty
In closing, Dr Daggubati noted that there are many forthcoming technologies for treating MR and mitral stenosis. He noted that the PARTNER trial is the current standard upon which other device approvals will be measured although trial methodologies and end points continue to evolve. He feels that improved imaging will accelerate the development of percutaneous MV therapies and that the interaction and collaboration between cardiothoracic surgery and cardiology will help to optimize the utilization of these novel therapies.
- © 2015 SAGE Publications