Summary
Acute myeloid leukemia, a common form of leukemia with a high mortality rate, is difficult to treat. In the SORAML trial, sorafenib, a multikinase inhibitor, prolonged event-free and relapse-free survival compared with a placebo in younger patients with acute myeloid leukemia. However, this benefit was accompanied by a higher rate of infections and bleeding.
- overall survival
- event free survival
- relapse free survival
- infection
- bleeding
- sorafenib
- placebo
- Study Evaluating Sorafenib Added to Standard Primary Therapy in Patients With Newly Diagnosed Acute Myeloid Leukemia Less Than 60 Years of Age
- SORAML
- NCT00893373
Christoph Röllig, MD, Universitätsklinikum Dresden, Dresden, Germany, presented the results of the Study Evaluating Sorafenib Added to Standard Primary Therapy in Patients With Newly Diagnosed Acute Myeloid Leukemia Less Than 60 Years of Age [SORAML; Röllig C et al. ASH 2014 (abstr 6)]. Although overall survival (OS) was not different compared with placebo, sorafenib significantly improved event-free survival (EFS) and relapse-free survival (RFS) with a cost of higher incidence of infections and bleeding events in younger patients with acute myeloid leukemia (AML).
AML is the most common form of leukemia in adults [National Cancer Institute. http://www.cancer.gov/cancertopics/pdq/treatment/adultAML/Patient/page1. Accessed December 23, 2014]. Survival rate for this disease continues to remain unsatisfactory, particularly among patients aged > 60 years. The significant genetic diversity and abnormality in AML even within a tumor of a single individual make it difficult to treat [Cancer Genome Atlas Research Network. N Engl J Med. 2013]. Kinase mutations are among the most frequently found genetic abnormalities. Mutations in the tyrosine kinase genes KIT and FLT3, and aberrant VEGF signaling through tyrosine kinases, play a critical role in AML biology. Sorafenib is a multikinase inhibitor with activity against several serine/threonine kinases and receptor tyrosine kinases.
SORAML was a randomized, multicenter, double-blind trial comparing sorafenib with placebo as an add-on to standard induction and consolidation treatment in 276 newly diagnosed AML patients aged 18 to 60 years with Eastern Cooperative Oncology Group (ECOG) performance 0 to 2, and adequate renal and liver function [Röllig C et al. ASH 2014 (abstr 6)]. Induction therapy consisted of 2 cycles of daunorubicin (60 mg/m2 days 3 to 5, plus cytarabine 100 mg/m2 cont. inf. days 1 to 7), followed by 3 cycles of high-dose cytarabine consolidation (3 g/m2 BID days 1, 3, and 5). Allogeneic stem cell transplantation was scheduled in the first complete remission. Patients were randomized (1:1) to receive sorafenib (800 mg/day) or placebo as an add-on to standard treatment. The primary end point was EFS. Secondary end points included RFS, OS, complete remission rate, and incidence of adverse events (AEs).
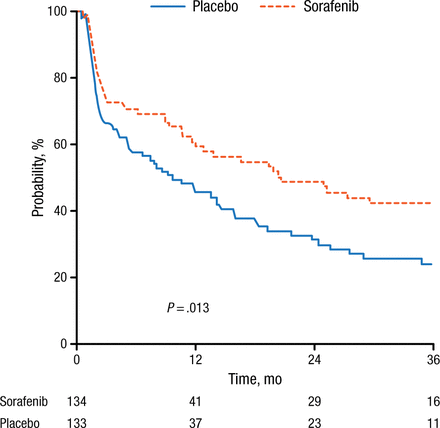
Demographic and disease characteristics were equally distributed between the two arms. The incidence of FLT3-internal tandem duplication (ITD) and NPM1 mutation was 17% and 33%, respectively. Median drug dose was approximately 16 200 mg with significantly less intake in the sorafenib arm, most likely due to side effects. Complete remission was evident in 60% of sorafenib-treated patients compared with 59% of placebo-treated patients (P = .764). EFS was 9.2 months and 20.5 months in the placebo and sorafenib groups, respectively, corresponding to a 3-year EFS of 22% vs 40% (P = .013; Figure 1).
Sorafenib Significantly Improves Event-Free Survival
Reproduced with permission from C Röllig, MD.
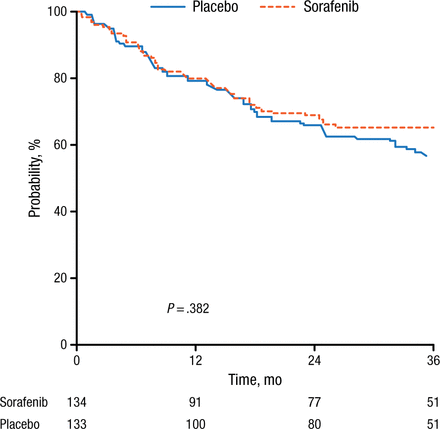
RFS was also significantly (P = .017) improved with sorafenib (56% after 3 years) treatment compared with placebo (38%), with a median RFS of 23 months with placebo compared with not yet reached after sorafenib treatment. At this point, there is no clear benefit for sorafenib treatment regarding OS (P = .382; Figure 2).
Sorafenib Does Not Improve Overall Survival Compared With Placebo
Reproduced with permission from C Röllig, MD.
In the 46 FLT3-ITD-positive patients, there was no difference in EFS, but there was a trend for prolonged RFS and OS in favor of sorafenib. The relative risk for the occurrence of grade ≥ 3 AEs was significantly higher for hand-foot syndrome, diarrhea, bleeding, rash, liver toxicity, and fever in the sorafenib arm compared with the placebo arm.
In this first randomized trial, sorafenib plus chemotherapy comprise a feasible treatment strategy in younger AML patients and are associated with improved outcomes.
- © 2014 SAGE Publications