Summary
This article discusses data on the effects of neoadjuvant and adjuvant chemotherapy (CT) and chemoradiotherapy (CRT) in patients undergoing surgery for gastroesophageal cancer. Evidence on the ways to improve morbidity and mortality associated with esophagectomy is also reviewed.
- Adjuvant/Neoadjuvant Therapy
- Gastrointestinal Cancers
- Head & Neck Cancers
- Oncology
In this session, the first 2 presenters discussed data on the effects of neoadjuvant and adjuvant chemotherapy (CT) and chemoradiotherapy (CRT) in patients undergoing surgery for gastro-esophageal cancer. The third presenter reviewed evidence on the ways to improve morbidity and mortality associated with esophagectomy.
NEOADJUVANT TREATMENT FOR GASTROESOPHAGEAL JUNCTION CANCER
Advantages of neoadjuvant CT for gastroesophageal junction (GEJ) cancer include downstaging of disease, treatment of micrometastases, evaluation of chemosensitivity, and improved safety of CT before postoperative morbidity, according to Marc Ychou, MD, Centre Régional de Lutte Contre le Cancer Val d'Aurelle, Montpellier, France. Disadvantages include disease progression before surgery, increased postoperative morbidity, and difficult primary tumor response assessment.
The Medical Research Council Adjuvant Gastric Infusional Chemotherapy [MAGIC; Cunningham D et al. N Engl J Med. 2006] and Fédération Nationale des Centres de Lutte contre le Cancer (FNCLCC)–Fédération Francophone de Cancérologie Digestive (FFCD) [Ychou M et al. J Clin Oncol. 2011] trials evaluated perioperative CT and surgery (CT + S) compared with surgery alone (S) in patients with GEJ.
Tumor downstaging was significantly greater in the MAGIC CT + S group versus S group (tumor, p = .009; nodes, p = .01), but there was no significant difference between the CT + S and S groups in the FNCLCC-FFCD study. The curative resection (R0) rate for the CT + S arm versus S arm was 69.3% versus 66.4% in the MAGIC study and 84% versus 74% (p = .04) in the FNCLCC-FFCD study. Five-year overall survival (OS) was better with CT + S versus S in the MAGIC trial (36% vs 23%; HR, 0.75; 95% CI, 0.60 to 0.93; p = .009) and FNCLCC-FFCD trial (38% vs 24%; HR, 0.69; 95% CI, 0.50 to 0.95; p = .02), respectively.
Tumor recurrence was reported in 39% versus 57% (MAGIC) and 55% versus 64% (FNCLCC-FFCD) of patients in the CT + S versus S groups, respectively.
A meta-analysis found that preoperative CT + S versus S for GEJ cancer was associated with longer overall survival (HR, 0.81; 95% CI, 0.73 to 0.89; p < .0001), longer disease-free survival (DFS), and higher R0 and downstaging rates [Ronellenfitsch U et al. Eur J Cancer. 2013].
Chris Willet, MD, Duke University Medical Center, Durham, North Carolina, USA, presented evidence for the use of neoadjuvant CRT in patients with GEJ cancer. He agreed that perioperative CT improves progression-free survival and OS. However, the impact of CT is modest, and the evidence on extent of resection is inconclusive. Furthermore, perioperative CT has no impact on locoregional failure (LRF) and distant metastasis (DM).
Neoadjuvant and adjuvant CRT have been shown to improve locoregional control and enhance survival (Table 1).
Adjuvant and Neoadjuvant CRT Studies
In the Intergroup 0116 trial, patients treated with adjuvant CRT versus S had improved OS and DFS and decreased relapse, LRF, and DM rates [MacDonald JS et al. N Engl J Med. 2001; Smalley SR et al. J Clin Oncol. 2012]. The CROSS trial demonstrated that neoadjuvant CRT improves R0 rates and OS and decreases LRF, DM, and peritoneal carcinomatosis [van Hagen P et al. N Engl J Med. 2012; Oppedijk V et al. J Clin Oncol. 2014]. In a comparison of neoadjuvant CT versus CRT in patients with esophageal cancer, preoperative CRT improved OS only in locally advanced esophagogastric adenocarcinoma [Stahl M et al. J Clin Oncol. 2009].
Dr Willet concluded that neoadjuvant CRT improves LRF and enhances survival. Neoadjuvant or perioperative CT improves survival by increasing the extent of resection and decreasing DM. Dr Willett concluded that neoadjuvant CRT and perioperative CT are effective.
STRATEGIES FOR IMPROVING MORBIDITY AND MORTALITY ASSOCIATED WITH ESOPHAGECTOMY
Christophe Mariette, MD, PhD, University Hospital of Lille, France, explored ways to improve surgery outcomes in patients with esophageal cancer. Appropriate patient selection is important to reduce morbidity and mortality associated with surgery. Relative contraindications include severe or multiple comorbidities, weight loss > 15% not corrected by nutritional support, grade ≥ 3 arteriopathy, and cirrhosis with no portal hypertension. In the past, age > 70 years was considered a contraindication, but now surgery is being performed successfully in patients in their 80s. Absolute contraindications include persistent weight loss > 20% despite nutritional support, World Health Organization performance status 3 or 4, respiratory insufficiency, decompensated cirrhosis or portal hypertension, and cardiac or renal insufficiency.
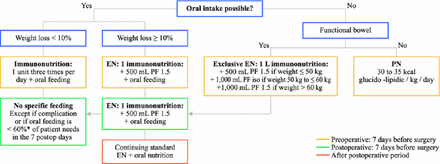
Preoperative conditioning includes tobacco and alcohol cessation, buccodental hygiene, and respiratory physiotherapy and rehabilitation. Malnutrition is present in 60% to 85% of surgery candidates. Patients who have lost weight have higher operative mortality and morbidity rates than patients who maintain their weight. Prof Mariette et al [Ann Surg Oncol. 2012] published guidelines for nutritional supplementation in surgery candidates (Figure 1). Weight loss < 10% should be treated with oral supplements, while enteral feeding is necessary for > 10% loss. Immunoenhanced nutrition decreases morbidity after gastrointestinal surgery [Cerantola Y et al. BR J Surg. 2012]. A Phase 3 trial is investigating immunoenhanced nutrition during the neoadjuvant phase [NCT01423799].
Algorithm for Preoperative and Postoperative Nutritional Supplementation
EN=enteral nutrition.
Reproduced from Mariette C et al. Surgery in esophageal and gastric cancer patients: what is the role for nutrition support in your daily practice? Ann Surg Oncol. 2012;19:2128–2134. With kind permission from Springer Science and Business Media.
Surgical technique affects the outcomes of surgery. An extended transthoracic esophagectomy with R0 resection and extended 2-field lymphadenectomy with examination of ≥ 23 lymph nodes can reduce recurrences and improve survival [Mariette C et al. Lancet Oncol. 2011; Peyre CG et al. Ann Surg. 2008]. Minimally invasive esophagectomy (MIE) has outcomes similar to open esophagectomy, with reduced blood loss, shorter hospital stay, and decreased morbidity and respiratory complications [Mariette C et al. Recent Results Cancer Res. 2010; Nagpal K et al. Surg Endosc. 2010]. A randomized trial of MIE (n = 56) versus open esophagectomy (n = 59) demonstrated reduced pulmonary infection in-hospital and within 2 weeks and no significant difference in nodes resected, R0 rates, and in-hospital and 30-day mortality [Biere SS et al. Lancet. 2012]. An ongoing multicenter randomized trial will assess morbidity, mortality, DFS, OS, and quality of life with laparoscopic MIE versus open esophagectomy [Briez N et al. BMC Cancer. 2011].
Enhanced recovery after surgery, or fast-track surgery, uses accelerated postoperative convalescence with a multimodal rehabilitation program to attenuate stress response and enable rapid recovery after surgery. Munitiz V et al [Br J Surg. 2010] found that patients with a clinical pathway for multidisciplinary postoperative management had significantly reduced pulmonary complications (14% vs 23%; p = .025), postoperative mortality (1% vs 5%; p = .010), and hospital stay (5 to 98 vs 8 to 106 days; p = .012) versus controls.
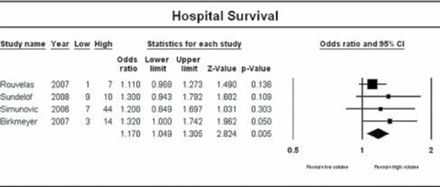
A systematic review and meta-analysis found that postoperative mortality and long-term survival (Figure 2) are reduced at high-versus low-volume centers [Wouters MW et al. Cancer. 2012].
Center Volume and Long-term Survival
Reproduced from Wouters MW et al. The volume-outcome relation in the surgical treatment of esophageal cancer. Cancer. 2012;118:1754–1763. With permission from John Wiley & Sons.
The evidence shows that surgical outcomes can be improved with appropriate patient selection and preconditioning. Outcomes are also improved with the enhanced recovery after surgery approach and in high-volume centers.
- © 2014 MD Conference Express®