Summary
The Society of Surgical Oncology and the American Society for Radiation Oncology have published a consensus guideline on margin widths in breast-conserving therapy, which is defined as surgical removal of the primary tumor with a margin of surrounding normal tissue followed by whole breast radiation therapy, as discussed in this article.
- Breast Cancer
- Radiology
- Radiation Therapy
- Oncology Guidelines
- Oncology
- Breast Cancer
- Radiology
- Radiation Therapy
- Oncology Guidelines
The Society of Surgical Oncology (SSO) and the American Society for Radiation Oncology (ASTRO) have published a consensus guideline on margin widths in breast-conserving therapy (BCT), which is defined as surgical removal of the primary tumor with a margin of surrounding normal tissue followed by whole breast radiation therapy (WBRT) [Moran MS et al. Ann Surg Oncol. 2014; Int J Rad Bio Physics. 2014; J Clin Oncol. 2014]. The guideline addresses the open question of the optimal width of negative margins to minimize local recurrence (LR) in patients with stage I and II invasive breast cancer, stated Meena S. Moran, MD, Yale University School of Medicine, New Haven, Connecticut, USA. The American Society of Breast Surgeons and the American Society of Clinical Oncology have also endorsed the guideline.
The objective of the multidisciplinary guideline panel was to identify a threshold width for the definition of a negative margin. Currently, there is significant variability in the definition of negative margins, and the current approach of using a random, predetermined narrow millimeter margin width has led to an excessive number of re-excisions, stated Dr Moran. The rates of re-excision after BCT range from 23% to 45% [Hadzikadic Gusic L et al. J Surg Oncol. 2014; McCahill LE et al. JAMA. 2012], and > 26 000 re-excisions are performed annually because of close margins [Greenup RA et al. Ann Surg Oncol. 2014], approximately 50% of which occur with no ink on tumor.
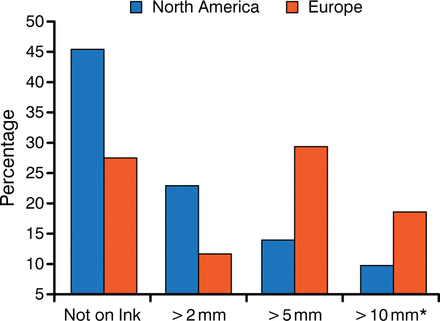
The substantial discordance amongst radiation oncologists and surgeons in defining negative margins is illustrated in Figure 1 [Taghian A et al. Ann Surg. 2005]. A reduction in LR was found to improve survival in the Early Breast Cancer Trialists' Collaborative Group meta-analysis [Darby S et al. Lancet. 2011], in contrast with phase 3 trial data that showed an equivalent effect on survival with breast-conserving surgery (BCS) and mastectomy. The variability in how a negative margin is defined may be due to the variability among the trials, because only one study (NSABP B-06) required a microscopic assessment and defined negative margin as no ink on tumor, whereas the others required only gross total resection.
Definition of Negative Margin in a Survey of North America and Europe
Adapted from Taghian A et al. Ann Surg. 2005.
The recommendations in the guideline are based on the findings of a study-level meta-analysis [Houssami N et al. Ann Surg Oncol. 2014], which comprised 33 studies identified by a systematic review commissioned by the guideline panel. Most of the studies were retrospective, which is a limitation of the scientific basis of the guideline. The inclusion criteria were studies of patients with stage I or II breast cancer who were treated with WBRT after BCS with > 4 years of follow-up and that reported patient age and LR in relation to margin status. Studies that included pure ductal carcinoma in situ (DCIS) or neoadjuvant chemotherapy were excluded. The study characteristics are summarized in Table 1.
Characteristics of Patients in Studies Included in the Meta-Analysis
Key findings from the meta-analysis included a median LR of 5.3% (1506 of the 28 162 patients with margin data) at a median follow-up of 6.6 years. The median recruitment year was 1990; thus, the majority of the patients were not treated with contemporary systemic therapy. Only 26% of patients had chemotherapy and 38% had endocrine therapy.
The SSO-ASTRO guideline defined a positive margin as ink on invasive cancer or DCIS and stated that it is associated with a ≥ 2-fold increase in ipsilateral breast tumor recurrence (IBTR) that is not nullified by the delivery of a boost dose of radiation or systemic therapy (endocrine, chemotherapy, biologic) or favorable biology. A negative margin was defined as no ink on tumor, and the guideline states that wider margin widths do not significantly reduce IBTR. Furthermore, the routine practice to obtain negative margin widths wider than no ink on tumor is not indicated.
The results of statistical modeling in the aforementioned meta-analysis provided the basis for these recommendations [Houssami N. Ann Surg Oncol. 2014]. A binary model of negative or combined positive or close margins found an OR of 1.96 (95% CI, 1.72 to 2.24) for IBTR with positive or close margins vs negative margins (OR, 1.0; P < .001), but was limited by the heterogeneity of the studies. An adjusted model that included only the studies (19 of 33) that provided precise margin width data found that compared with a negative margin, a positive (OR, 2.44; 95% CI, 1.97 to 3.03) or close (OR, 1.74; 95% CI, 1.42 to 2.15) margin increased the risk of IBTR. A model of pair-wise comparisons of 1-, 2-, and 5-mm margin widths and adjusted for a longer (8.7 years) median follow-up did not show a significant difference in LR with increasing width, nor was there any benefit in increasing margin distance for patients of younger age or those not receiving endocrine therapy or radiation therapy boost (Table 2).
Risk of Late Recurrence by Threshold Margin Distance and Clinical Variables
The consideration of other data regarding the current understanding of the influence of tumor biology on LR, the role of systemic therapy, findings from studies of nonsurgical management of microscopic nodal disease, and the reliability and reproducibility of margin measurements in millimeter increments led the guideline panel to conclude that there is no evidence to support wider margins for patients not receiving adjuvant systemic therapy, younger patients (who have an increased risk of LR), or for patients with different biologic sub-types. Recommendations related to radiation therapy, lobular histology, and extensive intraductal component are summarized in Table 3.
Considerations for Margin Widths
Breast cancer is a heterogeneous disease, and rather than focus on disease burden, it is time to move to a focus on the totality of breast cancer treatment, stated Monica Morrow, MD, Memorial Sloan Kettering Cancer Center, New York, New York, USA, and the margins guideline provides a starting point for progress. Dr Morrow emphasized that the guideline does not state that re-excision to obtain a wider margin is always inappropriate, but it emphasizes that rules that routinely require specific margin widths wider than no ink on tumor are not evidence based. The guideline recognizes that multiple factors beyond tumor burden influence LR. Furthermore, the wide variation in re-excision rates based on surgeon and practice characteristics suggests that there is a quality problem, not one of individualizing patient care, and because of the large volume, it is unrealistic to think that the tumor board will discuss each of the re-excision cases. Avoiding unnecessary re-excisions has the potential to save $30 million annually, as estimated using Medicare costs [Greenup RA et al. Ann Surg Oncol. 2014].
- © 2014 MD Conference Express®