Summary
Final overall survival and safety results of the phase 3 Everolimus and Octreotide in Patients With Advanced Carcinoid Tumor trial [RADIANT-3; NCT00412061] have bolstered previous findings that everolimus is effective and safe in the treatment of advanced pancreatic neuroendocrine tumors.
- Soft Tissue Cancers
- Oncology Clinical Trials
- Neuroendocrine Tumors
- Soft Tissue Cancers
- Oncology Clinical Trials
- Oncology
- Neuroendocrine Tumors
Final overall survival (OS) and safety results of the phase 3 Everolimus and Octreotide in Patients With Advanced Carcinoid Tumor trial [RADIANT-3; NCT00412061] have bolstered previous findings that everolimus is effective and safe in the treatment of advanced pancreatic neuroendocrine tumors (pNET).
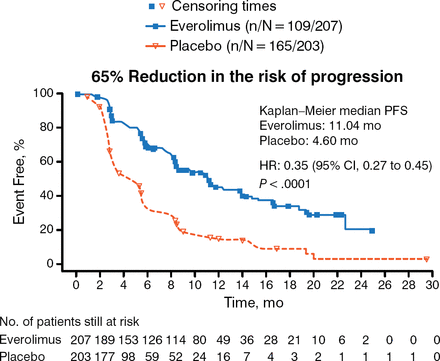
Prognosis is poor for patients with pNET, as the tumors are often advanced when diagnosed. In 2011, however, the RADIANT-3 trial investigators reported significantly improved median progression-free survival (PFS) in patients with advanced pNET treated using everolimus vs placebo (11.0 months vs 4.6 months) [Yao JC et al. N Engl J Med. 2011]. The present findings are the final OS and extended safety data including those from the open-label portion of the study.
RADIANT-3 investigators randomized 410 patients with advanced pNET in a 1:1 fashion to receive everolimus 10 mg/d (n = 207) or placebo (n = 203), both along with best supportive care, in the double-blind core phase of the study. In the open-label extension phase, crossover to the everolimus arm was allowed when disease progression occurred. The extension phase comprised 53 patients from the original everolimus arm and 172 crossover patients.
The primary end point was PFS according to RECIST 1.0 (Figure 1).
Primary End Point Data for RADIANT-3
The P value was obtained from a stratified one-sided log-rank test and the HR was obtained from a stratified unadjusted Cox model.
PFS, progression-free survival.
From Yao JC et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 2011;364:514–523. Copyright © 2011 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
Secondary end points included OS as of the March 5, 2014 cutoff date after 256 events (deaths). The OS analysis involved a stratified log-rank test in the intent-to-treat population of 410 randomized patients.
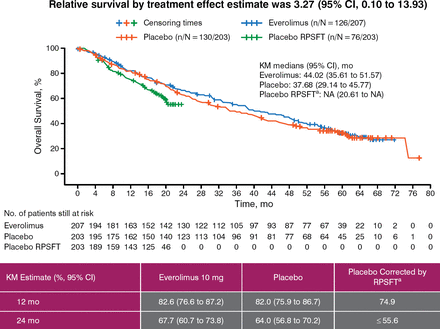
Of the 410 patients, 225 ultimately received everolimus; this included most of the patients initially randomized to the placebo arm (172 of 203, 85%). The median length of exposure to open-label everolimus following crossover was 44.0 weeks (range, 0 to 261 weeks) in patients originally randomized to placebo and 67.1 weeks (range, 1 to 189 weeks) in those originally randomized to the drug. At the OS cutoff, 126 of 207 patients (61%) in the everolimus arm and 130 of 203 patients (64%) in the placebo arm had died. In the latter, 23 of 130 deaths occurred before crossover. The median OS was 44.02 months (95% CI, 35.6 to 51.8 months) for the everolimus arm and 37.68 months (95% CI, 29.1 to 45.8 months) for the placebo arm. The results indicated a benefit for everolimus (HR, 0.94; 95% CI, 0.73 to 1.20) although statistical significance was not achieved (P = .30; significance boundary 0.0249) (Figure 2), likely due to crossover of 85% of patients originally randomized to placebo.
Final Overall Survival Analysis by RPSFT
Reproduced with permission from JC Yao, MD.
KM, Kaplan-Meier; RPSFT, rank-preserving structural failure time.
aReconstructed placebo data as if never treated with everolimus.
To account for the bias due to the large number of crossovers, the investigators conducted rank-preserving structural failure time (RPSFT) analysis, which corrects for the effect of crossover by estimating the multiplicative factor that estimates the effect of each day of everolimus treatment on overall survival and subsequently adjusts for the effect of everolimus received after crossover in the placebo arm.
The Kaplan-Meier and RPSFT-corrected survival estimates for five different time points are shown in Table 1. The hazard ratio was 0.90 (95% CI, 0.71 to 1.16), adjusted for baseline age, sex, region, and prior use of somatostatin analog.
Kaplan-Meier Overall Survival Estimate for Everolimus vs Placebo
The median OS of 44 months for everolimus-treated patients is the longest reported for progressive advanced pNET in a phase 3 study. The investigators noted that the improvement of 6.3 months in median OS vs placebo was clinically important.
- © 2014 MD Conference Express®