Summary
Considerable interest in preoperative systemic therapy (PST) for breast cancer has led to a large number of clinical trials investigating the potential of various preoperative treatments. A major driver in this effort has been the expectation that positive preoperative results would translate into improvements in disease-free survival and overall survival. This article discusses chemotherapy, the impact of PST to enable breast-conserving therapy, radiation issues after PST, as well as considerations for radiation therpay treatment of inflammatory breast cancer.
- Adjuvant/Neoadjuvant Therapy
- Breast Cancer
- Oncology
- Adjuvant/Neoadjuvant Therapy
- Breast Cancer
PREOPERATIVE SYSTEMIC THERAPY: WHY AND WHEN?
Considerable interest in preoperative systemic therapy (PST) for breast cancer has led to a large number of clinical trials investigating the potential of various preoperative treatments. A major driver in this effort has been the expectation that positive preoperative results would translate into improvements in disease-free survival (DFS) and overall survival (OS). Speakers at a session of the Presidential Symposium at the 2014 American Society for Radiation Oncology Annual Meeting discussed how the use of PST in breast cancer can impact subsequent surgery and radiation therapy (RT).
Leading off the session was Eric P. Winer, MD, Dana-Farber Cancer Institute, Boston, Massachusetts, USA. Dr Winer first discussed chemotherapy, using data from a meta-analysis indicating no survival advantage or disadvantage for pre- vs postoperative chemotherapy [Mauri D et al. J Natl Cancer Inst. 2005], adding that the same lack of advantage or disadvantage should also be the case for the sequencing of hormonal therapy relative to surgery. Dr Winer also described studies whereby PST led to improvements in pathologic complete response (pCR) but not to improvements in DFS or OS [Rastogi P et al. J Clin Oncol. 2008], and discussed the importance of tumor type in such correlations [Cortazar P et al. Lancet. 2014].
Of note, since 2012, a US Food and Drug Administration guidance has allowed drug manufacturers to submit pCR data as part of their submission request for accelerated approval. Dr Winer noted that this guidance may have been overbroad, given the fact that sometimes there is a paucity of DFS or OS results to accompany the pCR data. He presented results of the phase 3 ALTTO trial [[NCT00490139; Piccart-Gebhart MJ. ASCO. 2014] on use of trastuzumab and lapatinib, which failed to show significant improvement in DFS and OS in breast cancer despite improved pCR, to highlight the point that results from clinical trials of preoperative therapy may not be sufficient to support drug approval. Dr Winer said that the patients appropriate to receive PST are those who need downstaging to minimize surgery or those who choose to enter clinical trials. Dr Winer concluded by saying that although there is clinical value to PST in reducing local therapy and that PST is a potentially powerful research tool, pCR data generated from PST trials are not ready to inform drug approval.
SURGICAL ISSUES AFTER PST
Judy C. Boughey, MB, BChir, Mayo Clinic, Rochester, Minnesota, USA, was the next speaker, and she described the impact of PST to enable breast-conserving therapy (BCT). The volume of tissue resected for T2 and T3 tumors in patients who received preoperative chemotherapy was shown to be nearly one-half that of those who received chemotherapy postoperatively (Table 1), a reduction anticipated to improve cosmetic outcomes. There was no difference in rates of re-excision or of recurrence between preoperative and postoperative chemotherapy groups [Boughey JC et al. Ann Surg. 2006].
Volume of Tissue-Resected T2 and T3 Tumors After Breast-Conserving Surgery and Preoperative Chemotherapy
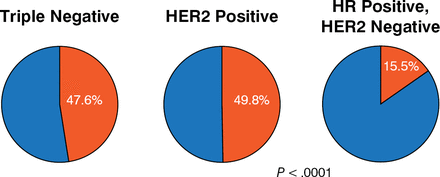
As in the previous talk, the impact of tumor biology on pCR was addressed. Data from the ACOSOG Z1071 clinical trial [Boughey JC et al. Ann Surg. 2014] included 694 evaluable patients treated with PST from 2009 to 2011 who had either BCT or mastectomy at the patient's and/or physician's choice. One-fourth of patients had triple-negative (TN) breast cancer and nearly one-half had hormone receptor (HR)-positive, human epidermal growth factor 2 (HER2)-negative disease, whereas 30% had HER2-positive tumors. In terms of pCR rates, TN and HER2-positive patients showed rates of 48% and 50%, respectively, whereas HR-positive, HER2-negative patients had a much lower pCR rate of 16% (Figure 1).
Pathologic Complete Response Rates by Tumor Type
HER2, human epidermal growth factor 2; HR, hormone receptor.
Source: Boughey JC et al. Ann Surg. 2014.
Reproduced with permission from Boughey JC, MB, BChir.
In looking at the procedures that these patients underwent, rates of mastectomy were significantly higher (P < .0001) in HR-positive, HER2-negative patients with the much lower pCR rates. However, a significant portion of patients who did achieve pCR chose to undergo mastectomy. Dr Boughey commented that this result indicated an opportunity for improving imaging procedures to predict response and changing the way that patients are counseled and educated regarding surgical options.
RADIATION ISSUES AFTER PST
Moving from issues of surgery after PST to the topic of RT following PST, Lori J. Pierce, MD, University of Michigan Medical School, Ann Arbor, Michigan, USA, also highlighted the ability of PST to downstage disease in the breast and the axillary nodes. Addressing how the administration of PST and the body's response should impact recommendations for locoregional radiation, Dr Pierce said that for patients with locally advanced disease, the answer is “not at all.” However, the answer is less clear for patients with early stage breast cancer.
A review of data on recurrence following neoadjuvant chemotherapy from the NSABP-18 and NSABP-27 clinical trials [Mamounas EP et al. J Clin Oncol. 2012], in which patients with BCT were allowed RT for the breast only and no regional RT, showed increased locoregional response rates (LRRs) in cases of residual disease in the lymph nodes and/or breast and increased LRRs in younger women. Mastectomy patients had even more restrictions on radiation with no postsurgery RT allowed. These patients also displayed increased LRRs with residual disease in the breast or lymph nodes and for tumors > 5 cm, but had lower LRRs for cases of pCR in the breast and nodes.
INFLAMMATORY BREAST CANCER: UNIQUE RADIOTHERAPY AND BIOLOGICAL CONSIDERATIONS
The final speaker in the session was Wendy Woodward, MD, PhD, University of Texas MD Anderson Cancer Center, Houston, Texas, USA. She did not have a focus on PST, but rather discussed considerations for RT treatment of inflammatory breast cancer (IBC), a rare and aggressive disease in which cancer cells block lymph vessels in the skin of the breast. The disease is so named because this blockage often causes the breast to look swollen and red (ie, with an inflamed appearance). IBC is responsible for 1% to 5% of all breast cancers diagnosed in the United States. Most cases are invasive ductal carcinomas that develop first from cells lining the milk ducts, and then spread. An adequate, homogeneous skin dose of RT is needed, with a bolus that should be individualized to the extent of the patient's skin disease and any acute effects. There should be big margins around all surgical bed changes and skin involvements.
A 79-gene signature developed from IBC samples classifies 25% of the tumors in The Cancer Genome Atlas as “IBC-like” [Robertson FM et al. Springer Plus. 2013]. Because of an enrichment of stem cells in the normal cells of patients with TN breast cancer, it has been hypothesized that these stem cells persist in the breast after pregnancies not followed by breastfeeding, later contributing to TN disease. In support of this hypothesis, Dr Woodward showed unpublished xenograft data indicating that mesenchymal stem cells promoted clinical signs of IBC in mice and presented a model for the development of a premalignant field within a postpregnancy, nonbreastfeeding breast that could also promote IBC features.
- © 2014 MD Conference Express®