Summary
This article discusses current perspectives on the management of patients with locally advanced rectal cancer (LARC). Specific topics include radiotherapy in LARC, adjuvant and neoadjuvant chemotherapy, and the surgical treatment of LARC.
- Gastrointestinal Cancers
- Reproductive Cancers
- Oncology
- Gastrointestinal Cancers
- Reproductive Cancers
In an education session, Andres Cervantes-Ruiperez, MD, PhD, University of Valencia, Valencia, Spain; Robert Glynne-Jones, MD, Mount Vernon Hospital, Northwood, United Kingdom; and Cornelis J. H. van de Velde, MD, PhD, Leiden University Medical Center, Leiden, Netherlands, discussed current perspectives on the management of patients with locally advanced rectal cancer (LARC).
According to Prof Cervantes-Ruiperez, the management of rectal cancer has changed from a surgical model to a multidisciplinary treatment model. This has been facilitated by improvements in management options in LARC, including the evolution of total mesorectal excision (TME) surgery, optimal staging by magnetic resonance imaging (MRI), pathologic evaluation of the quality of the surgery, and preoperative radiotherapy (PRT) or chemoradiation therapy (CRT).
RADIOTHERAPY IN LARC
Dr Glynne-Jones emphasized that radiotherapy is an important component of the multimodal treatment of patients with rectal cancer, particularly if the circumferential resection margin is threatened. For resectable cancers, he discussed its role in reducing recurrence in high-risk cases, adding that it is less useful in low-risk cancers because the risk of recurrence is already so low.
He noted that 3D external beam radiotherapy is the most widely used type of radiation therapy in rectal cancer. While historical data advise a dose of at least 30 Gy, he added that evidence-based guidelines advocate 45 to 50 Gy, usually in combination with capecitabine or 5-FU.
Short-course PRT and preoperative CRT are both acceptable options for resectable rectal cancer, but Dr Glynne-Jones stressed that their routine use is not yet universal. In one study of patients with rectal cancer, neoadjuvant CRT did not increase survival (P = .960), local control (P = .170), or late toxicity (P = .360) when compared with short-course PRT alone [Bujko K et al. Br J Surg. 2006]. A recent study also showed no significant difference in recurrence-free survival (P = .47) or overall survival (OS; P = .62) between short- and long-course neoadjuvant radiotherapy for T3-stage rectal cancer [Ngan SY et al. J Clin Oncol. 2012]. In the landmark German l CAO/ARO/AIO-94 trial [Sauer R et al. N Engl J Med. 2004], local disease recurrence was significantly reduced (P = .006) with preoperative CRT compared with postoperative CRT. Acute (P = .001) and late adverse events were also significantly reduced (P = .01), although there was no difference in OS between the 2 groups (P = .80).
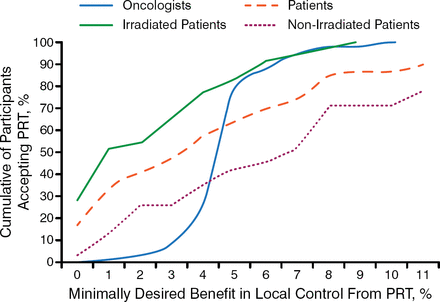
However, Prof Glynne-Jones highlighted the conflict between evidence-based medicine and individualized selection, emphasizing the importance of patient participation in treatment decision making, particularly because the range of required benefit from PRT varies widely between patients and oncologists (Figure 1) [Pieterse AH et al. Br J Cancer. 2007].
Patient and Oncologist Treatment Preferences Differ
PRT, preoperative radiotherapy.
Reprinted by permission from Macmillan Publishers Ltd: British Journal of Cancer. Pieterse AH et al. Benefit from preoperative radiotherapy in rectal cancer treatment: disease-free patients' and oncologists' preferences. 2007;97:717–724, Copyright 2007.
He also discussed some of the problems of defining in whom radiotherapy should be used. Although MRI is the most accurate method of staging and assessing the relationship of the tumor to the mesorectal fascia, not all centers use it to plan rectal cancer treatment, so optimal decisions can be missed if it is not performed or not recorded. Additionally, not all surgeons are performing high-quality resections via TME or abdominoperineal excision of the rectum. Consequently, he stressed that radiotherapy will always be necessary to compensate for poor surgery.
ADJUVANT AND NEOADJUVANT CHEMOTHERAPY
Prof Cervantes-Ruiperez reviewed the current evidence for postoperative adjuvant chemotherapy in rectal cancer, which supports its use for some patients with LARC, including those at risk after direct surgery, those at high risk after neoadjuvant chemotherapy (NACT), or those with locally advanced cancer who responded well to CRT. Among the studies that he presented were the Quasar study, which showed a small but significant improvement (P = .05) in 5-year survival associated with postoperative adjuvant chemotherapy [Quasar Collaborative Group. Lancet. 2007].
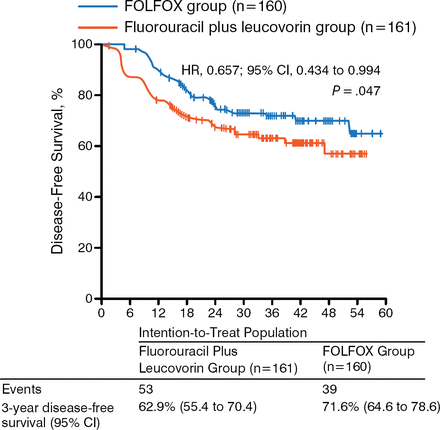
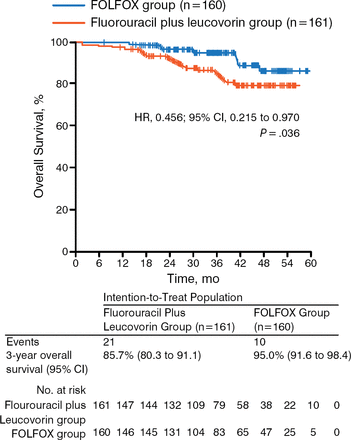
While a recent randomized phase 3 trial showed no benefit in disease-free survival (DFS; P = .56) or OS (P = .75) of postoperative adjuvant capecitabine and oxaliplatin compared with observation in LARC [Glynne-Jones R et al. Ann Oncol. 2014], the study could not complete accrual and therefore lacked the statistical power to show a potential effect of NACT on survival. The ADORE trial, however, showed that adjuvant FOLFOX improves DFS (P = .047; Figure 2) and OS (P = .036; Figure 3) in LARC when compared with fluorouracil plus leucovorin [Hong YS et al. Lancet Oncol. 2014]. These results support the need for additional trials that assess the role of adjuvant chemotherapy in LARC.
Results of the ADORE Trial: Disease-Free Survival
Reprinted from The Lancet Oncology, 15:1245–1253, Hong YS et al, Oxaliplatin, fluorouracil, and leucovorin versus fluorouracil and leucovorin as adjuvant chemotherapy for locally advanced rectal cancer after preoperative chemoradiotherapy (ADORE): an open-label, multicentre, phase 2, randomized controlled trial, Copyright 2014, with permission from Elsevier.
Results of the ADORE Trial: Overall Survival
Reprinted from The Lancet Oncology, 15:1245–1253, Hong YS et al, Oxaliplatin, fluorouracil, and leucovorin versus fluorouracil and leucovorin as adjuvant chemotherapy for locally advanced rectal cancer after preoperative chemoradiotherapy (ADORE): an open-label, multicentre, phase 2, randomized controlled trial, Copyright 2014, with permission from Elsevier.
Prof Cervantes-Ruiperez emphasized that although NACT is an important option in treating rectal cancer, it is still predominantly experimental and must be validated in additional randomized phase 3 trials in patients with MRI-defined, high-risk LARC. He shared data from a pooled analysis of EXPERT and EXPERT-C, the 2 largest trials of neoadjuvant capecitabine and oxaliplatin (CAPOX), followed by CRT, TME, and adjuvant CAPOX, with or without cetuximab, in patients with MRI-defined, high-risk LARC. The radiologic response was 62% following NACT and 80% after CRT. After a median follow-up of 69 months, 5-year local progression-free survival (PFS), distant PFS, PFS, and OS were 94%, 79%, 70%, and 73%, respectively [Sclafani F et al. J Clin Oncol. 2014 (abstr 3575)].
Moving forward, the ongoing RAPIDO trial [NCT01-558921] is designed to test whether short-course radiation, followed by up-front chemotherapy before surgery, improves 3-year DFS in patients with LARC when compared with conventional chemoradiation.
SURGICAL TREATMENT OF LARC
According to Prof van de Velde, the Beyond TME Collaborative group concluded that achieving an R0 resection with free resection margins is the most important goal in these patients [Beyond TME Collaborative. Br J Surg. 2013]. Given the heterogeneity of these cases, he added that a variety of surgical solutions may be considered to accomplish this goal, so the procedure must be personalized to suit the individual patient's clinical situation.
Discussing the evolution of surgical approaches to LARC in recent years, he noted that although the extended resection technique is used, it is hazardous and should be performed only in centers of excellence. Robotic surgery is in a learning curve and aims to reduce technical difficulties associated with performing standard laparoscopic surgery within the narrow pelvic cavity, offering similar operative time and quality of mesorectal excision, with a reduced duration of hospital stay [Baik SH et al. Surg Endosc. 2008]. The ongoing prospective randomized controlled ROLARR trial [Collinson FJ et al. Int J Colorectal Dis. 2012] aims to provide a comprehensive assessment of robotic-assisted and standard laparoscopic surgery for the curative resection of rectal cancer.
Prof van de Velde noted that near-infrared fluorescence imaging represents another exciting development with the potential to dramatically change current staging methods in the management of patients with LARC. In Europe, audit of the treatment results of rectal cancer has been one of the most important developments, leading to initiation of the European Registration of Cancer Care to improve the quality of care for patients with colon and rectal cancer [van de Velde CJ et al. Eur J Cancer. 2014].
Despite advances in the surgical treatment of LARC, however, he emphasized that there is still room for improvement, especially in a multidisciplinary setting and in particular with respect to enhancing the ability to identify nerves and avoid damaging them. Surgical techniques must also be refined to improve organ preservation, concluded Prof van de Velde.
- © 2014 MD Conference Express®