Summary
Treatment of advanced genitourinary cancers remains challenging. This article provides updates in genitourinary oncology addressed some important questions in the field that need answers to improve patient outcomes. Specific topics include clinical trial data for the currently approved agents, unresectable bladder cancer, as well as the treatment of metastatic renal cell carcinoma.
- Reproductive Cancers
- Gastrointestinal Cancers
- Reproductive Cancers
- Oncology
- Gastrointestinal Cancers
Treatment of advanced genitourinary cancers remains challenging. A session on updates in genitourinary oncology addressed some important questions in the field that need answers to improve patient outcomes.
Metastatic, castration-resistant prostate cancer (mCRPC) is clinically and pathologically heterogeneous between patients as well as within a given patient. CRPC is driven by androgen receptor (AR) signaling. AR alterations are selected during therapy. Resistance can be driven by androgen production in the testis, adrenal glands, or prostate tumor; AR overexpression; AR splice variations; AR mutants; signaling cross-talk; and upregulation of AR cofactors [Hu R et al. Expert Rev Endocrinol Metab. 2010; Heinlen C, Chang C. Endocr Rev. 2004].
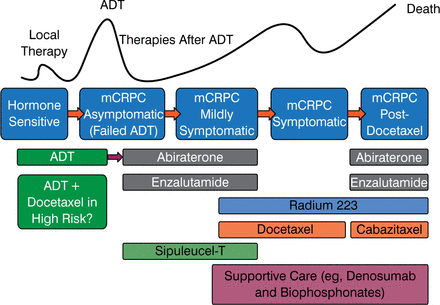
The current treatment paradigm for prostate is evolving, as shown in Figure 1. There are a lot of drugs available, but the optimal order in which to use them is not known, nor is the best combination or sequence known.
Current Treatment Paradigm for Prostate Cancer Is Evolving
ADT, androgen deprivation therapy; mCRPC, metastatic castration-resistant prostate cancer.
Reproduced with permission from CN Sternberg, MD.
Cora N. Sternberg, MD, San Camillo and Forlanini Hospitals, Rome, Italy, reviewed clinical trial data for the currently approved agents. There are no prospective trial data available concerning sequencing of the newer agents. In addition, clinical or molecular predictive factors are urgently needed. Ongoing phase 3 trials, however, are beginning to address these gaps.
One exciting development is the discovery of AR splice variants. Of the 20 or so described, AR-V7 is the most common, and patients who express it have a 0% prostate-specific antigen (PSA) response to either abiraterone or enzalutamide [Antonarakis ES et al. N Engl J Med. 2014]. In those patients without AR-V7, PSA responses are 68% and 53%, respectively. Splice variants were measured in circulating tumor cells in the blood so they are easier to measure than AR nuclear expression, which requires a bone marrow biopsy. If these results are validated, it may guide future treatment.
Unlike prostate cancer, bladder cancer, particularly when unresectable or inoperable, has not been the subject of many large, randomized, controlled clinical trials despite more than half a million new cases of bladder cancer yearly worldwide. Peak incidence is around the seventh decade of life, and 20% of patients are older than 80 years, so bladder cancer will become an enormous challenge as the population ages.
Maria De Santis, MD, Center for Oncology and Hematology, Vienna, Austria, discussed unresectable bladder cancer, calling the outcome of patients with this disease dismal, in part because there is no salvage therapy for surgery that leaves positive margins.
Old trial data suggest it may be possible to treat inoperable bladder cancer with chemotherapy alone. Unpublished data from recent European Organization for Research and Treatment of Cancer (EORTC) subgroup analyses for patients treated with various types of combination chemotherapy demonstrated a clinical, not pathologic, complete response (CR) rate of up to 23% and an overall response rate of nearly 50%, but this involves very small numbers of patients. In addition, the relapse rate is high after CR. Therefore, chemotherapy alone is not recommended as primary therapy for localized bladder cancer, according to the European Association of Urology 2014 guidelines [Babjuk M et al. Eur Urol. 2014].
There has been no head-to-head comparison of radiotherapy with surgery, and trials of each modality have occurred in different patient populations. Five-year survival rates appear to be similar, however, so radiotherapy could be an alternative to radical cystectomy in patients who refuse or are unfit for surgery. Hypofractionated radiotherapy has been shown in older retrospective trials in small numbers of patients to be well tolerated and an option for palliation of pain and hematuria in muscle-invasive bladder cancer in frail elderly patients.
Chemoradiation is also feasible, and it provides a palliative benefit and, in rare instances, long-term disease-free survival. It has been studied, however, only in early-phase trials in small numbers of patients who were negatively selected because they were unfit for surgery or their tumors were not amenable to surgery, and these trials had very different end points.
Bernard Escudier, MD, Institute Gustave Roussy, Villejuif, France, discussed the treatment of metastatic renal cell carcinoma (mRCC). He pointed out that genomic classification lags behind that of other tumor types, although genomic signatures are being developed.
Risk assessment is important in mRCC. Prof, Escudier recommended using the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) criteria for risk evaluation [Heng DYC et al. J Clin Oncol. 2009]. The 6 risk factors include (1) Karnofsky performance status < 80%, (2) hemoglobin less than the lower limit of normal, (3) time from diagnosis to treatment < 1 year, (4) corrected calcium greater than the upper limit of normal (ULN), (5) platelets > ULN, and (6) neutrophils > ULN. Patients with none of these factors have a favorable prognosis, those with 1 or 2 have an intermediate prognosis, and those with 3 or more have a poor prognosis.
Treatment recommendations from the European Society For Medical Oncology (ESMO) 2014 Clinical Practice Guidelines for RCC are summarized in Table 1 [Escudier B et al. Ann Oncol. 2014].
Renal Cell Carcinoma: ESMO Clinical Practice Guidelines for Diagnosis, Treatment, and Follow-Up
The role of nephrectomy is not clear, although patients with poor IMDC risk factors should not undergo surgery.
If a CR is achieved, which is not common, it is not clear what should be done. Prof. Escudier recommends that if CR is achieved with systemic treatment only, that treatment should be continued for 2 to 3 months to confirm CR, then discontinued. If CR was achieved with systemic plus local treatment, treatment should stop after the local treatment. A “drug holiday” is feasible, particularly in patients with indolent, stable disease. The appropriate time to switch to second-line therapy is not known. Prof. Escudier suggested that if a patient has primary refractory disease (ie, no response to a drug after 3 months of therapy), if an active second-line treatment is available, it should be used.
The future of RCC treatment may lie with new agents, such as checkpoint inhibitors (eg, nivolumab) that target programed death-1 (PD-1) receptors, as well as with cMet inhibitors. Other needs in RCC are the development of biomarkers to select the best therapy for patients, and determination of the best combinations of therapies.
To improve treatment of genitourinary cancers, more large prospective clinical trials are needed to test new and combination therapies. In addition, translational research is needed for the identification of biomarkers and establishment of prognostic genetic profiles to personalize therapy for patients with mCRPC, unresectable bladder cancer, and RCC.
- © 2014 MD Conference Express®