Summary
Invasive fungal disease warrants special considerations in special populations, such as children, patients being cared for in the intensive care unit (ICU), and patients who have undergone transplantation. This article discusses issues in pediatric candidiasis, fungal infections in the intensive care unit, as well as how to effectively use the clinical microbiology laboratory to address fungal infections in solid organ and bone marrow transplant patients.
- Fungal Infections
- Infectious Disease
- Fungal Infections
Invasive fungal disease warrants special considerations in special populations, such as children, patients being cared for in the intensive care unit (ICU), and patients who have undergone transplantation. William J. Steinbach, MD, Duke University School of Medicine, Durham, North Carolina, USA, discussed issues in pediatric candidiasis.
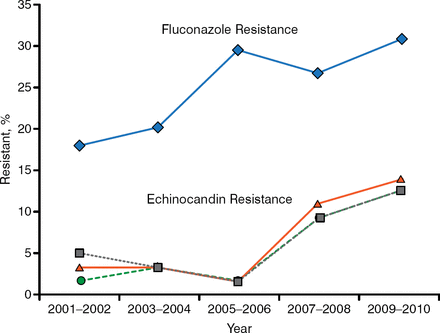
In pediatric patients, Candida glabrata resistance to echinocandins and fluconazole is rising. The SENTRY global surveillance study conducted between 2006 and 2010 demonstrated fluconazole resistance in 9.7% of isolates; 98.8% of those were not susceptible to voriconazole, and 11.1% were also resistant to ≥ 1 echinocandin [Pfaller MA et al. J Clin Microbiol. 2012]. In contrast, echinocandin resistance was not detected between 2001 and 2004 in 110 C. glabrata isolates that were resistant to fluconazole. In a study conducted at Duke, among 293 cases of C. glabrata candidemia in adults between 2001 and 2010, echinocandin and fluconazole resistance increased by 7.4% and 12.1%, respectively (Figure 1) [Alexander BD et al. Clin Infect Dis. 2013]. In a retrospective study of 344 pediatric cases of candidemia, independent risk factors for pediatric C. glabrata or Candida krusei infection were age > 2 years, exposure to fluconazole in the past 15 days, or recent surgery in the past 15 days [Prasad PA et al. J Pediatric Infect Dis Soc. 2013].
Resistance rates of Candida glabrata to echinocandins and fluconazole
Reproduced from Clin Infect Dis, Alexander BD, Increasing Echinocandin Resistance in Candida glabrata: Clinical Failure Correlates With Presence of FKS Mutations and Elevated Minimum Inhibitory Concentrations, 2014;56:1724–1732, Copyright 2014, with permission from Infectious Diseases Society of America.
Another issue is that there are no pediatric-specific diagnostic recommendations for invasive candidiasis (IC) in the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guideline [Hope WW et al. Clin Microbiol Infect. 2012]. In adults, 3 daily blood cultures for a total of 40 to 60 mL are recommended [Cuenca-Estrella M et al. Clin Microbiol Infect. 2012]; however, this guideline cannot be followed for pediatric patients due to lower blood volume. In addition, the diagnostic sensitivity of blood cultures is not high; for example, the sensitivity for all candidiasis is estimated to be 50% in adults, and the sensitivity is unknown in children [Clancy CJ et al. Clin Infect Dis 2013]. Dr Steinbach suggested that the sensitivity is likely lower because of the lower blood volume in children. In addition, there are few studies of molecular diagnostics for Candida in children. Only case reports or case series have been published using the Fungitell (1→3)-β-glucan assay; there are no published reports for use of the Platelia Candida mannan antigen and anti-mannan antibody assay, polymerase chain reaction (PCR) for Candida, and the T2Candida test in children. In a meta-analysis of the Fungitell (1→3)-β-glucan assay in adults, the sensitivity for 2 consecutive tests was 49.6% (95% CI, 34 to 65.3), and the specificity was 98.9% (95% CI, 97.4 to 99.5) [Lamoth F et al. Clin Infect Dis. 2012]. The positive predictive value was 83.5%, and the negative predictive value was 94.6%. In a study of 120 nonimmunocompromised pediatric patients with a mean age of 9.2 years, the mean value of beta-glucan was higher (68 pg/mL) than what was previously reported for adults (48 pg/mL) [Smith PB et al. Clin Vaccine Immunol. 2007]. Several other small studies or case reports have been published; 1 study of 61 neonates found that the optimal cutoff value was 125 pg/mL [Goudjil S et al. J Matern Fetal Neonatal Med. 2013].
The 2012 ESCMID guideline for the diagnosis and treatment of Candida diseases includes a discussion on the treatment of IC in children [Hope WW et al. Clin Microbiol Infect. 2012]. In nonneutropenic adults, an echinocandin is recommended for patients who have moderate to severe disease or had recent exposure to an azole agent, whereas fluconazole is recommended for patients who are less critically ill and had no recent azole exposure. Dr Steinbach highlighted that treatment of Candida in children generally follows this recommendation, but there is a lack of evidence to support its use. Dr Steinbach outlined what he does in practice. For a fluconazole loading dose, he administers 25 mg/kg on day 1, followed by 12 mg/kg/d. In children on extracorporeal membrane oxygenation, he administers a 35 mg/kg loading dose, followed by 12 mg/kg/d.
There are dosing issues in children. For caspofungin, a body surface area dosing scheme is used; for micafungin, age is important to consider because the drug's clearance increases substantially in younger patients, particularly neonates; and there has been only 1 pharmacokinetic study in children for anidulafungin. In this study, anidulafungin was cleared more quickly in children according to a nonlinear association with weight; therefore, higher doses are likely needed as weight decreases to achieve equivalent drug exposure [Hope WW et al. Antimicrob Agents Chemother. 2007]. Voriconazole pharmacokinetics also differs in children. The adult bioavailability is 96%, whereas the pediatric bioavailability is 44.6% [Karisson MO et al. Antimicrob Agents Chemother. 2009]. For oral voriconazole, lower peak concentrations were observed in children ages 2 to 5 years compared to children 6 to 11 years; 1 cohort received 4 mg/kg IV q12h, then 6 mg/kg IV q12h, and then 4 mg/kg orally q12h, and the other cohort received 6 mg/kg IV q12h, then 8 mg/kg IV q12h, and then 6 mg/kg orally q12h [Walsh TJ et al. Antimicrob Agents Chemother. 2010]. In a study of pediatric patients in Japan, children age < 3 years needed a greater oral dose compared with children ≥ 3 years [Shima H et al. Pediatr Blood Cancer. 2010]. Based on the available data, the optimal dosing of IV and oral voriconazole is dependent on age (Table 1).
Dosing of Voriconazole for Pediatric Patients
Luis Ostrosky-Zeichner, MD, University of Texas Health Science Center, Houston, Texas, USA, discussed fungal infections in the intensive care unit (ICU). According to the EPIC II survey, 17% of infections in the ICU population are Candida, and 1.4% are Aspergillus [Vincent JL et al. JAMA. 2009].
A major problem related to fungal infections in the ICU is diagnostic capabilities. Traditional diagnostic techniques such as blood cultures are not reliable, because 50% of patients with Candida will have a negative blood culture for Candida, and most blood cultures will test negative for Aspergillus and Mucor in patients with invasive fungal infections (IFIs) [Ostrosky-Zeichner L. Am J Med. 2012]. Tissue biopsies and other types of cultures are not always feasible, and contamination can be a problem. To maximize the sensitivity of blood culturing, it is important to use the latest, most advanced techniques, including those that are automated.
Markers such as Candida-specific antigens, including mannan antigen and anti-mannan antibody, are primarily used in Europe, but they have been shown to have 75% to 100% specificity and 52% to 83% sensitivity for candidemia [Arendrup MC et al. Clin Microbiol Infect. 2010]. Candida PCR is a promising methodology, and a meta-analysis of Candida PCR concluded that the more stringent the criteria for the positive and negative controls, the better the results of this technique to diagnose IC [Avni T et al. J Clin Microbiol. 2011]. Another molecular-based diagnostic assay uses the T2 detection technology, which has been reported to have extremely high sensitivity and specificity for several Candida species to diagnose candidemia [Beyda ND et al. Diag Microbiol Infect Dis. 2013]. A large, multicenter validation study evaluating the T2 detection technology is expected to be published in late 2014 or early 2015. A meta-analysis showed that the β-D-glucan marker was a good marker for IFI, with sensitivity and specificity rates of 76.8% and 85.3%, respectively, to distinguish proven or probable IFI vs no IFI [Karageorgopoulos DE et al. Clin Infect Dis. 2011].
A diagnostic marker for Aspergillus is galactomannan, which has been demonstrated to have a sensitivity rate of 85% in patients with proven fungal disease and a specificity of 70% [Marr KA et al. Clin Infect Dis. 2005]. For Aspergillus PCR, a recent meta-analysis demonstrated that it has a sensitivity of about 90% and specificity in the 80% range for invasive aspergillosis [Arvanitis M et al. J Clin Microbiol. 2014]. High-resolution computed tomography (CT) can have good sensitivity as well, and classic signs of invasive Aspergillus infection include the presence of a “halo,” crescent, nodules, ground glass, and cavitation [Gotway MB et al. J Comput Assist Tomogr. 2002]. Dr Ostrosky-Zeichner stated that we need to move away from treating full-blown disease and move toward preventing fungal infection and engaging in preemptive treatment of early disease.
Kimberly E. Hanson, MD, MHS, University of Utah, Salt Lake City, Utah, USA, used case studies to illustrate how to effectively use the clinical microbiology laboratory to address fungal infections in solid organ and bone marrow transplant patients. In 1 case, a 29-year-old man with aplastic anemia presented with painful skin lesions and sinusitis. Histopathologic analysis of skin biopsies taken from the lesions showed invasive fungal elements and septated fungal hyphae. Dr Hanson pointed out that, microscopically, it is impossible to distinguish between different fungal genera, because septated hyphae with acute angle branching are a common characteristic of many species. Panfungal PCR followed by DNA sequencing of tissue samples can be used to differentiate among species. In terms of growing cultures, clues for clinical significance include fungal elements on direct staining, a site of isolation from the host, the same fungus is isolated from multiple specimens, and multiple colonies grow from the same specimen.
To identify the species, DNA sequencing can be used; however, the regions most commonly used in sequencing are highly conserved in Fusaria, and therefore dedicated PCR targets are needed. It is important to identify the specific species because the susceptibility profile can be different. One study found that the minimum inhibitory concentration (MIC) at 50% ranged between 2 and 4 μg/mL for amphotericin, and between 4 and 8 μg/mL for voriconazole, and it was > 16 μg/mL for posaconazole (Table 2) [Espinel-Ingroff A et al. J Clin Microbiol. 2007]. Antifungal susceptibility testing is important to determine treatment options, to rule out secondary resistance after prolonged exposure to the drug, and to rule out resistance when the infection is refractory to treatment.
Antifungal Susceptibility Profile for Fusarium spp
In patients who have undergone solid organ transplants, the most common Candida species that cause infection are Candida albicans (46%) and C. glabrata (25%) [Lockhart SR et al. J Clin Microbiol. 2011]. Fluconazole resistance is common in C. glabrata (23%) and C. krusei (100%), whereas about 1% of C. albicans is resistant. Methods for rapid identification of Candida include peptide nucleic acid fluorescent in situ hybridization, multiplex reverse transcription-PCR, and matrix-assisted laser desorption ionization-time-of-flight mass spectrometry. Rapid identification can decrease the time to optimal therapy and, in some cases, decrease overall costs.
In conclusion, rapid and early identification of fungal species is important to improve treatment decisions and to administer optimal therapy to patients with invasive fungal disease. Although there are limitations to many of the current diagnostic techniques, newer techniques have demonstrated promising sensitivity and specificity.
- © 2014 MD Conference Express®