Summary
The first randomized, controlled trial to show a survival benefit of an antifungal treatment in HIV-infected patients with cryptococcal meningitis was completed this year in Vietnam [ISRCTN 95123928].
- Infectious Disease Clinical Trials
- HIV & AIDS
- Fungal Infections
The first randomized, controlled trial to show a survival benefit of an antifungal treatment in HIV-infected patients with cryptococcal meningitis was completed this year in Vietnam [ISRCTN 95123928]. Results were presented by Jeremy N. Day, MD, Oxford University Clinical Research Unit, Wellcome Trust Major Overseas Programme Vietnam, in collaboration with colleagues from the Hospital for Tropical Diseases, Ho Chi Minh City, Vietnam.
The study compared three induction-phase treatment strategies that are currently recommended by the Infectious Disease Society of America [Perfect JR et al. Clin Infect Dis 2010]. Although combination therapy with flucytosine is considered first-line therapy, a mortality benefit over other regimens has not been shown in a randomized, controlled trial. Also, there are distinct disadvantages to flucytosine use—namely expense, toxicity, and poor availability in areas with high cryptococcal disease rates.
Dr. Day and his colleagues were interested in whether combining antifungal therapies in the induction phase of treatment would offer a survival advantage when compared with amphotericin monotherapy, the standard practice in Vietnam.
Enrolled patients presented with a syndrome that was consistent with cryptococcal meningitis and microbiological evidence of Cryptococcus in the CSF and/or blood. All patients were >14 years of age and HIV-positive. Patients with prior history of cryptococcal infection or prior antifungal treatment (>3 days) were excluded. Patients were randomly assigned to receive one of three possible induction treatments: amphotericin B 1 mg/kg/day monotherapy for 4 weeks (Arm I, the standard of care in Vietnam); amphotericin B 1 mg/kg/day plus flucytosine 100 mg/kg/day for 2 weeks (Arm II); or amphotericin B 1 mg/kg/day plus fluconazole 400 mg twice daily for 2 weeks (Arm III; Table 1). The coprimary endpoint was mortality at 2 and 10 weeks. Secondary endpoints included survival to 6 months and disability at 70 days and 6 months.
Study Design.
The intent-to-treat (ITT) population comprised 298 patients, predominantly male, with a median age of 28 years. Approximately 30% had some level of impaired consciousness, reflected by a Glasgow coma score of <15. All patients underwent lumbar puncture, which revealed elevated CSF opening pressure (>18 cm/CSF) in over two-thirds of patients and high yeast burdens (median 5.9 log 10 CFU/mL).
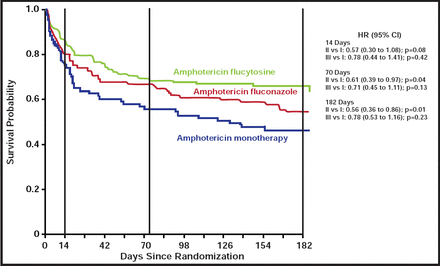
Compared with amphotericin monotherapy, the amphotericin+flucytosine combination was associated with a significantly reduced hazard of death by both Day 70 [HR, 0.61; 95% CI, 0.39 to 0.97; p=0.04] and Day 182 [HR, 0.56; 95% CI, 0.36 to 0.89; p=0.01] (Figure 1). Amphotericin B, combined with fluconazole, offered no survival advantage compared with amphotericin monotherapy. After adjusting for fungal burden and Glasgow coma score at study entry, the hazard of death by 6 months was also significantly higher among amphotericin-fluconazole-treated patients versus those who received amphotericin-flucytosine (adjusted HR for all-cause mortality, 1.81; 95% CI, 1.14 to 2.88; p=0.01). The death rate at 70 days was 30% for patients who were on combination therapy with flucytosine versus 44% for those who were on monotherapy. Rates of adverse events between the two combination regimens were comparable and included anemia, neutropenia, and renal impairment.
Kaplan-Meier Curve of Survival Outcomes Among ITT Population.
Significantly improved survival noted among patients treated with flucytosine-containing combination therapy (Arm II, green line) compared with amphotericin monotherapy (Arm I, blue line) at 70 days and 182 days.
Reproduced with permission from J. Day, MD.
Dr. Day concluded by saying that in light of this research, improving access to amphotericin and flucytosine in regions where cryptococcal disease is prevalent, such as southeast Asia and Africa, has the potential to significantly reduce the global burden of deaths due to this devastating disease.
- © 2011 MD Conference Express