Summary
Patients with invasive fungal disease and comorbid conditions such as hematologic malignancy and uncontrolled malignancy were successfully treated with the novel triazole antifungal agent isavuconazole, regardless of minimum inhibitory concentrations required by baseline Aspergillus spp isolates. This article presents data according to type of malignancy from the Isavuconazole (BAL8557) for Primary Treatment of Invasive Aspergillosis trial [SECURE; NCT00412893].
- Infectious Disease Clinical Trials
- Fungal Infections
- Infectious Disease Clinical Trials
- Fungal Infections
- Infectious Disease
Patients with invasive fungal disease (IFD) and comorbid conditions such as hematologic malignancy (HM) and uncontrolled malignancy (UncCA) were successfully treated with the novel triazole antifungal agent isavuconazole, regardless of minimum inhibitory concentrations (MICa) required by baseline Aspergillus spp isolates. Andrew J. Ullmann, MD, Julius Maximilians University, Würzburg, Germany, presented data according to type of malignancy from the Isavuconazole (BAL8557) for Primary Treatment of Invasive Aspergillosis trial [SECURE; NCT00412893].
IFD is a challenge, particularly in immunocompromised patients [Leventakos K et al. Clin Infect Dis 2010; Kontoyiannis DP et al. Clin Infect Dis 2010; Pappas PG et al. Clin Infect Dis 2010], and patients with UncCA [Bohme A et al. Ann Hematol 2009] and HM [Pagano L et al. Haematologica 2006]. In addition, mortality rates remain high in populations such as organ or hematopoietic stem cell transplant recipients who have invasive aspergillosis (IA) [Baddley JW et al. Clin Infect Dis 2010]. The broad-spectrum triazole antifungal agent, isavuconazole, demonstrated efficacy against multiple pathogens, including Aspergillus spp, Candida spp, Cryptococcus spp, and Mucorales in vitro, as well as IA, invasive candidiasis, mucormycosis, and cryptococcosis in animal models [Lepak A et al. Antimicrob Agents Chemother 2013; Lepak A et al. Antimicrob Agents Chemother 2013; Luo G et al. Antimicrob Agents Chemother 2014]. The overarching purpose of the SECURE trial was to evaluate the safety and efficacy of isavuconazole in patients with IFD [Maertens J et al. ECCMID 2014 0230a]; the purpose of this analysis was to evaluate the outcomes of isavuconazole treatment in patients with UncCA who participated in the SECURE trial.
In the multicenter, noninferiority, Phase 3 SECURE trial, 516 patients (intention-to-treat population) age ≥ 18 years with proven or probable IFD were randomly assigned to receive 200 mg IV TID isavuconazole for 2 days followed by 200 mg (IV or oral) QD or standard-dose voriconazole. The primary end point of all-cause mortality at day 42 was 22% and 25% in the isavuconazole and voriconazole arms, respectively, with drug-related adverse events occurring more frequently in the voriconazole arm (60 vs 40%). Secondary end points included success rate, adverse events, and other safety parameters.
In the SECURE trial, 178 patients had UncCA, and they were evenly split between the isavuconazole and voriconazole arms. In patients with UncCA, the all-cause mortality at day 42 was 21% and 22% in patients who received isavuconazole compared with voriconazole, respectively, with a mean difference of −0.5 (95% CI, −9.6 to 8.6). The overall response at the end of the trial (EOT) was 36% and 34% in the isavuconazole and voriconazole arms, respectively, with a mean difference of −2.2 (95% CI, −17.4 to 13.0). Patients without UncCA demonstrated lower rates of all-cause mortality at day 42 in the intention-to-treat population; however, patients with UncCa taking isavuconazole demonstrated lower rates of all-cause mortality compared with the voriconazole arm. EOT overall response was greatest in patients infected with mold species not otherwise specified and Aspergillus spp only compared with non-Aspergillus spp only or positive serum for galactomannan.
Treatment-emergent adverse events (TEAEs) were similar among both treatment arms in patients with UncCA; however, fewer patients in the isavuconazole arm (40%) experienced drug-related adverse events compared with those treated with voriconazole (60%).
In conclusion, Prof Ullmann indicated that patients with UncCA demonstrated greater mortality rates in both treatment arms compared with patients without UncCA; however, patients treated with isavuconazole experienced fewer drug-related adverse events.
Kieren A. Marr, MD, Johns Hopkins University, Baltimore, Maryland, USA, presented an analysis of patients with HM from the SECURE trial. Out of the 516 patients in the intention-to-treat population, 433 had HM, of which 217 had proven or probable IFD.
In patients with HM, all-cause mortality at day 42 was 22% and 24% in the isavuconazole and voriconazole arms, respectively, with a mean difference of −1.5 (95% CI, −13.7 to 10.7). All-cause mortality was lowest in patients with acute myeloid leukemia compared with those with UncCA, acute lymphocytic leukemia, neutropenia, or allogenic hematopoietic stem cell transplant. At EOT, the overall response rate was 39% and 34% in the isavuconazole and voriconazole arms, respectively, with a mean difference of −5.0 (95% CI, −18.8 to 8.9).
TEAEs were similar between both treatment arms in patients with HM. 97% of patients in the ISA arm and 99% of patients in the VRC arm developed at least 1 TEAE; however, drug-related adverse events were greater in patients with HM who were treated with voriconazole (59%) compared with isavuconazole (44%). Significantly fewer (P < .05) TEAEs of the skin, eye, and hepatobiliary system organ classes were observed in the ISA arm of the study. In the modified intent-to-treat population, 97% of the ISA patients and 98% of the VRC patients reported TEAEs. Among this population, significantly fewer (P < .05) TEAEs were observed in the ISA arm than the VRC arm, including cardiac, eye, renal and urinary, and psychiatric disorders.
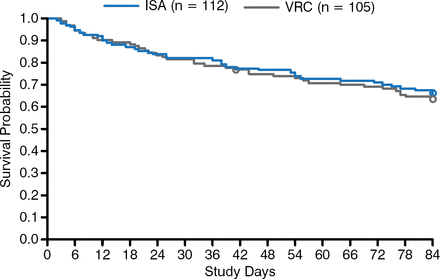
In conclusion, Dr Marr indicated that the data suggest that treatment of IFD with isavuconazole results in comparable efficacy outcomes as with voriconazole, but with a lower risk of drug-related adverse events (Figure 1).
Survival Rates of Patients With Hematologic Malignancy and Invasive Fungal Disease
ISA, isavuconazole; VRC, voriconazole.
Reproduced with permission from K Marr, MD.
David Andes, MD, University of Wisconsin, Madison, Wisconsin, USA, presented data from an analysis of outcomes by MICs from the SECURE trial. In this study, Aspergillus spp isolates, the majority of which were A. fumigatus, were collected at baseline from patients enrolled in the SECURE trial for analysis of MIC values.
In patients treated with isavuconazole, the MIC50 and MIC90 values for isavuconazole were 1 and 4 μg/mL (range, 0.25 to 32). and for voriconazole they were 1 and 2 μg/mL (range, 0.12 to 32). In patients treated with voriconazole, the MIC50 and MIC90 values were 1 and 2 μg/mL (range, 0.25 to 4) for isavuconazole and voriconazole, respectively. There was no association between baseline MIC values and all-cause mortality at day 42 or successful overall response at EOT (Table 1).
All-Cause Mortality Stratified by Microorganism and Minimum Inhibitory Concentrations, Through Day 42
In conclusion, Dr Andes indicated that the data from the MIC analysis of isolates from the SECURE trial demonstrated that successful outcomes were achieved with a range of MIC values, including higher values. In addition, patient outcomes were not associated with MIC value. Dr Andes pointed out that the MIC analysis was limited by its small sample size.
- © 2014 MD Conference Express®