Summary
In this session, presenters focused on patient perspectives in the context of clinical trials, patient-reported outcomes, and regulatory science in the medical device industry. Presenters discussed collaborative research, centers that evaluate medical devices, patient surveys, and patient preferences in nephrology.
- Treatments
- Treatments
- Nephrology
In this session, presenters focused on patient perspectives in the context of clinical trials, patient-reported outcomes, and regulatory science in the medical device industry. Presenters discussed collaborative research, centers that evaluate medical devices, patient surveys, and patient preferences in nephrology.
Collaborative research is conducted with patients, rather than “on” them, and a partnership is formed that enables the shared control of the production, use, and dissemination of knowledge gained from research. Celeste Castillo Lee, University of Michigan Health System, Ann Arbor, Michigan, USA, presented the patient perspective of clinical trials. When patients are partners in research, they can help to define research questions, design surveys, formulate recommendations, and define characteristics of a patient-centered trial.
Carolyn Y. Neuland, PhD, US Food and Drug Administration (FDA), Silver Spring, Maryland, USA, discussed the mission of the Center for Devices and Radiologic Health (CDRH), which is to protect and promote public health. The CDRH's vision is that all patients have access to medical devices that are safe, effective, and of high quality. When evaluating devices, the CDRH takes into account patient preferences, which include anecdotal comments submitted to the FDA, opinions expressed on social media, qualitative ad hoc surveys, and patient-reported outcome instruments. Patient preference is defined as qualitative or quantitative assessments of the relative desirability or acceptability of attributes that differ among alternative diagnostic or therapeutic strategies.
An example of patient preferences and benefit-risk guidance is the tolerance of risk [FDA. http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm267829.htm. Accessed December 12, 2014]. Some patients have a high tolerance for risk (ie, accepting great risk to gain a small benefit), whereas others are unwilling to take on much risk for a small benefit. The FDA strives to determine what patients believe to be a meaningful benefit during the process of evaluating the efficacy of a device. To facilitate this process, the patient preference initiative was developed to request patient views of a medical device. One pilot study involves a survey to determine the feasibility of eliciting and use of patient preferences in the topic of obesity and weight loss. The results from this study helped to determine minimum clinically meaningful weight loss from the patient perspective, and it allowed researchers to determine the values that patients assign to risks vs benefits.
In 2012, the Medical Device Innovation Consortium (MDIC) was formed in partnership with the FDA with a mission to improve regulatory science in the medical device industry. To achieve this mission, the MDIC aims to coordinate the development of tools, methods, and resources for the management of the life cycle of a medical device. One of MDIC's projects is called the Patient-Centeredness and Benefit-Risk Assessment Project, in which there are strategies to assess the patient perspective of benefits and risks associated with a device.
Ronald D. Perrone, MD, Tufts Medical Center, Boston, Massachusetts, USA, discussed the challenges and opportunities in patient-reported outcome (PRO) measures for kidney disease. PROs are based on data, such as survey data that are directly reported by the patient. There are multiple tools that can be used, such as generic instruments that are useful to evaluate the overall health status and disease-specific instruments that evaluate factors that are more directly affected by the disease state. For example, a survey was used to evaluate patient outcomes related to pain in autosomal dominant polycystic kidney disease (ADPKD) [Bajwa ZH et al. Kidney Int. 2004]. In the survey, about 60% of the respondents reported abdominal pain as measured by the visual analog scale.
In the COHORT study [Rizk D et al. Clin J Am Soc Nephrol. 2009], a survey that included a physical component summary (PCS) and a mental component summary (MCS) found no significant difference from the general population. However, patients who received pain medication 30 days before the survey had a lower PCS score. In the HALT PKD study [Miskulin DC et al. Am J Kidney Dis. 2014], patients with ADPKD completed the Short-Form-36 health survey (SF-36) and the HALT PKD Pain Questionnaire. Fifty percent of patients reported back pain, with 20% experiencing it often, usually, or always. In addition, patients with a lower estimated glomerular filtration rate were more likely to report that pain affected their daily lives.
In 2013, the FDA published a report of PROs in the areas of anemia secondary to chronic kidney disease, ADPKD, and nephrotic syndrome [Perrone RD et al. Am J Kidney Dis. 2013]. The purpose of the report was to outline how the FDA reviews and evaluates PRO instruments that are used in clinical trials as data to support their claims of a given medical product. The end point model was created to help develop PROs, in which the concept such as the indication or supportive concept is defined and related to an end point. For example, for an indication concept of treatment of ADPKD symptoms, the patient-reported end point could be the total ADPKD symptoms score.
Another strategy to elicit feedback from patients is to hold a focus group. This allows patients affected by the disease to express their beliefs and perspectives regarding multiple aspects of the disease and its treatment. For example, focus groups that included 117 patients with ADPKD were held in the United States, Europe, and Japan that allowed patients to relate emotional and physical impairments caused by the disease [ERA-EDTA. 2011]. After 25% of the focus groups were held, saturation was achieved, meaning that all of the emotional and physical concepts of ADPKD were identified.
For the quantitative measurement of PROs, cross-sectional and longitudinal studies can be conducted.
Francesca Tentori, MD, Arbor Research Collaborative for Health, Ann Arbor, Michigan, USA, and Vanderbilt University Medical Center, Nashville, Tennessee, USA, described patient preferences in nephrology. Patient preferences are important because lower MCS and PCS scores are associated with mortality [Mapes DL et al. Kidney J. 2003]. Yet, the practice of routinely evaluating the quality of life (QOL) of patients varies widely across countries participating in the Dialysis Outcomes Practice Patterns Study (DOPPS).
There are limitations of tools that are presently used to assess patient-centered outcomes. For example, many tools are time-consuming; therefore, only selected patients complete the assessment, and they may be too burdensome for many facilities to use on a routine basis.
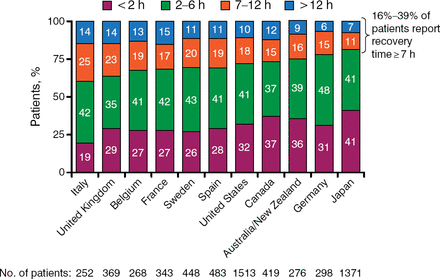
The DOPPS study [Rayner HC et al. Am J Kidney Dis. 2014] evaluated patient-reported recovery time in 23 patients who underwent frequent hemodialysis (HD) and 22 controls. The study validated the recovery time tool, because the score was associated with fatigue, dialysis stress, disease stress, SF-36 subscales, and health utilities. The tool found that recovery time varied across multiple countries, with 19% to 41% stating that recovery time was < 2 hours and 35% to 48% indicating that recovery time was 2 to 6 hours (Figure 1). In addition, lower MCS and PCS scores were associated with longer time to recovery after HD.
Patient-Reported Recovery Time After Hemodialysis
Adapted from Am J Kid Diseases, Vol 64, Rayner HC et al, Recovery Time, Quality of Life, and Mortality in Hemodialysis Patients: The Dialysis Outcomes and Practice Patterns Study (DOPPS), Pages 86–94, Copyright (2014), with permission from National Kidney Foundation, Inc.
QOL and patient satisfaction are likely not the only factors that patients with kidney disease are concerned about. The EPOCH-RRT study [NCT01952600] sought to identify factors that are most important to patients with kidney disease who require HD through patient interviews. These data will be compared with data gathered from the PDOPPS study, which will assess outcomes in patients undergoing peritoneal dialysis.
In conclusion, patient preferences can be used in clinical trials to assess the effectiveness of a medical device through various patient-reported instruments. The FDA has integrated patient preferences into their assessment of medical devices to determine meaningful benefits of a product [Perrone RD et al. Am J Kidney Dis. 2013].
- © 2015 MD Conference Express®