Summary
Autosomal dominant polycystic kidney disease is the fourth-leading cause of end-stage renal disease in the United States. Just-released results from the HALT Progression of Polycystic Kidney Disease (HALT PKD) Trial sought to determine whether BP control or RAAS blockade slows the progression of renal disease, as discussed in this article.
- Cystic Kidney Diseases
- Nephrology Clinical Trials
- Renal Failure
- Cystic Kidney Diseases
- Nephrology Clinical Trials
- Renal Failure
- Nephrology
Autosomal dominant polycystic kidney disease (ADPKD) is the fourth-leading cause of end-stage renal disease (ESRD) in the United States. It is characterized by renal cyst growth with increased kidney volume, resulting in activation of the renin-angiotensin-aldosterone system (RAAS), the development of early hypertension, and renal failure. Just-released results from the HALT Progression of Polycystic Kidney Disease (HALT PKD) Trial indicate that rigorous blood pressure (BP) control is associated with a slower increase in total kidney volume (TKV) compared with a standard BP goal and dual blockade of the RAAS with an angiotensin-converting enzyme inhibitor (ACE-I) and an angiotensin receptor blocker (ARB) was not more effective than ACE-I monotherapy.
The HALT PKD Trial sought to determine whether BP control or RAAS blockade slows the progression of renal disease. The BP portion [Study A; NCT00283686; Schrier RW et al. N Engl J Med. 2014] was reported by Arlene B. Chapman, MD, Emory University School of Medicine, Atlanta, Georgia, USA [Schrier RW et al. N Engl J Med. 2014]. Vincente E. Torres, MD, PhD, Division of Nephrology and Hypertension, Mayo Clinic, Rochester, Minnesota, reported on RAAS blockade [Study B; NCT01885559; Torres VE et al. N Engl J Med. 2014].
The hypothesis for Study A was that low (95-110/60-75 mm Hg) vs standard (120-130/70-80 mm Hg) BP control will reduce the rate of disease progression as measured by percentage change in TKV. For both Study A and Study B, a second hypothesis was that dual blockade of the RAAS with an ACE-I and an ARB will reduce the rate of disease progression vs an ACE-I alone.
STUDY A
The primary end point in Study A was percentage change in TKV. Secondary end points were the slope of change in estimated glomerular filtration rate (eGFR), urine albumin, and aldosterone excretion; left ventricular mass index (LVMI); renal blood flow and renal vascular resistance (RVR); the frequency of all-cause and cardiovascular hospitalizations; quality of life; pain; and PKD-related symptoms. Study A included healthy patients 15 to 49 years of age with a baseline eGFR > 60 mL/min. Patients were randomized to either standard (n = 284) or low BP control (n = 274) and then to either lisinopril (ACE-I) plus telmisartan (ARB) or lisinopril plus a placebo.
Participants were mean age of approximately 36 years and were mostly (> 90%) white. Between 71% and 79% were mutation-type PKD1. Mean body mass index was approximately 27 kg/m2, and mean eGFR was 92 mL/min/m2. Patients in the low BP group experienced greater decreases in home systolic/diastolic BP by the end of study (difference, 13.4/9.3 mm Hg). Urinary aldosterone excretion decreased significantly for both groups, but there were no significant differences (difference, −1.19; 95 CI, −3.07 to 0.60; P = .19) between the 2 groups. Annualized percentage change in TKV was 5.67% in the low BP group and 6.57% in the standard BP group (P = .006). This resulted in a 14.2% difference in the reduction in TKV growth at the end of the study. There was no significant difference in percentage change in TKV between cohorts receiving lisinopril plus telmisartan and lisinopril plus placebo.
In the first 4 months during titration, the low BP group had a significant decline in eGFR of −3.1 while the standard BP group had a significant increase of 0.5. The differences were significant (P < .001). There was a marginally positive slope difference in the long term (P = .05), although overall the slopes for the 2 groups did not differ.
Urinary albumin excretion declined significantly more in the low vs standard BP control group (−3.77% vs +2.43%; P < .001). LVMI decreased significantly more in the low BP group vs the standard BP group (−1.17 g/m2/year vs −0.57; P < .001). RVR increased in the standard BP group but did not change in the low BP group. The mean duration of follow-up exceeded 5 years. The rate of adverse events was low and did not differ between the 2 groups.
STUDY B
Study B comprised ADPKD patients (n = 485) 18 to 64 years of age (mean, 49 years) with an eGFR of 25 to 60 mL/min/1.73 m2 (mean, 48–49). Participants were randomized to receive telmisartan plus lisinopril (n = 244) or lisinopril plus placebo (n = 242). Doses were adjusted to achieve a BP of 110/70 to 130/80 mm Hg. The primary end point in Study B was a composite of time to death, ESRD, or 50% reduction in eGFR. Secondary end points were the slope of change in eGFR, urine albumin, and aldosterone excretion; the frequency of all-cause and cardiovascular hospitalizations; quality of life; pain; and PKD-related symptoms. Patients were followed for 5 to 8 years (mean, 5.2 to 6.7 years).
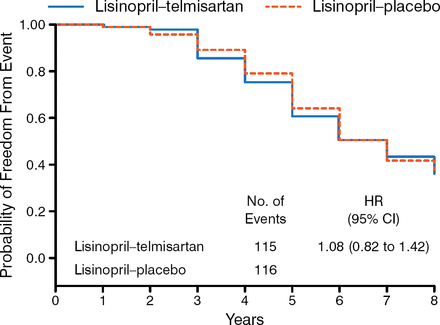
Equal proportions of men and women participated in Study B. Participants had a mean age of 49 years and were mostly white (≥ 93%), with a mean eGFR of 48 to 49. There was no significant difference between the study groups in the incidence of the primary outcome either as a composite or individually (Figure 1). There were also no treatment differences in the change in slope for eGFR and urine albumin excretion rates, frequency of polycystic kidney disease-related symptoms, quality of life, or pain.
Primary Outcome
Adapted from New England Journal of Medicine, Torres VE et al, Angiotensin blockade in late autosomal dominant polycystic kidney disease. 371:2267–2276. Copyright © 2014 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
Both dual blockade and monotherapy with an ACE-I produced similar BP control, while both treatments lowered urinary aldosterone excretion similarly.
The investigators concluded that dual therapy was as effective and safe as monotherapy in ADPKD and chronic kidney disease patients in stages 1 to 3. Adding an ARB to ACE-I does not confer additional benefits.
- © 2015 MD Conference Express®