Summary
Bone morphogenetic proteins (BMPs) were used with increasing frequency in spine surgery after 2002, but their use has decreased as concerns have developed about possible cancer risk and complications. In this session, presenters discussed the evidence regarding BMPs and their associated cancer risk, other possible complications, and the patterns of BMP use since 2002.
- Spine Conditions Orthopaedic Procedures
- Spine Conditions
- Orthopaedics
- Orthopaedic Procedures
Bone morphogenetic proteins (BMPs) were used with increasing frequency in spine surgery after 2002, but their use has decreased as concerns have developed about possible cancer risk and complications. In this session, presenters discussed the evidence regarding BMPs and their associated cancer risk, other possible complications, and the patterns of BMP use since 2002.
BMPS AND CANCER RISK
Wellington K. Hsu, MD, Northwestern University, Evanston, Illinois, USA, first reviewed the usage and function of BMP. Although the use of recombinant human BMP (rhBMP) increased prior to the issuance of a US Food and Drug Administration (FDA) warning [Schultz DG. FDA Public Health Notification. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PublicHealthNotifications/ucm062000.htm. Accessed November 2014], usage dropped more rapidly after a review article suggested increased cancer risk for rhBMP-2 [Carragee EJ et al. Spine J. 2011].
Dr Hsu stated that although there was no evidence of a mechanism by which BMPs could cause cancer through induction, there was potential that BMPs could cause cancer through promotion (tumorigenesis) or by enabling metastasis. For example, one study showed that BMPs critically influenced the formation of osteoblastic lesions in metastatic prostate cancer [Feely BT et al. J Bone Miner Res. 2005].
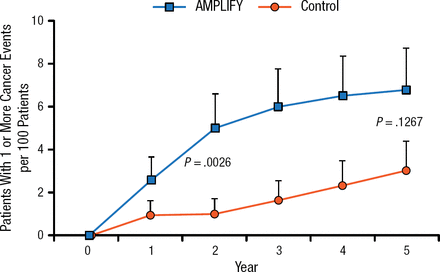
Despite the linkage of rhBMP-2 to cancer in a critical review by Carragee and colleagues [Spine J. 2011], Dr Hsu noted that the study had limitations. In particular, the study did not specifically look at the incidence of Surveillance Epidemiology and End Results cancers but rather included all cancers and all stages of cancers. Another study also demonstrated increased cancer risk with AMPLIFY, a high-dose rhBMP-2 product that is not currently approved by the FDA (Figure 1). AMPLIFY has a relatively high dose of rhBMP-2 and no dose-dependent effects were noted [Carragee EJ et al. J Bone Joint Surg Am. 2013]. When a particular agent is associated with cancer, a dose-dependent effect is expected.
Comparison of Patients With Cancer Events Using AMPLIFY vs Control
Adapted from Carragee EJ et al. Cancer Risk After Use of Recombinant Bone Morphogenetic Protein-2 for Spinal Arthrodesis. J Bone Joint Surg Am. 2013;95:1537–1545. With permission from The Journal of Bone and Joint Surgery, Inc.
To summarize these 2 studies, Dr Hsu stated that while there is evidence that AMPLIFY may be linked to cancer, it is not clear how this translates to risks for lower-dose, FDA-approved rhBMP-2 (eg, INFUSE), and it will be important to control for cancer types in future studies.
Dr Hsu also reviewed data from the Yale University Open Data Access (YODA) Project, in which 2 sites independently reviewed data on rhBMP-2 and cancer risk [Fu R et al. Ann Intern Med. 2013; Simmonds MC et al. Ann Intern Med. 2013]. One group was at the Oregon Health and Science University, Portland, Oregon, USA, while the other was at the University of York, York, United Kingdom. Both sites reported low cancer risks, that Medtronic had underreported risks, and that the cancers seen were heterogeneous. However, only 1 site found an increased risk for cancer at 24 months (RR, 3.45; 95% CI, 1.98 to 6.00). The other site found a nonsignificantly higher cancer rate in the rhBMP-2 group. Dr Hsu described differences in the data analysis (for example, the University of York group excluded pre-existing cancers and 1 trial) that were responsible for these findings and concluded that more research was needed.
In contrast to these results, another study indicated a lower risk for Surveillance, Epidemiology, and End Results cancers [Kelly MP et al. J Bone Joint Surg Am. 2014]. Dr Hsu noted that other studies have suggested similar results but have limitations (eg, using diagnoses only rather than observations of patients). Thus, it is unclear whether there is a dose-dependent relationship.
Dr Hsu published a review [J Bone Joint Surg Am. 2014] with recommendations about when to use rhBMP-2 during spine surgery. He suggested that rhBMP-2 use should be considered on a case-by-case basis, based upon the wishes of the patient following counseling and whether the risk of complications is reasonable. He advises that for patients with a history of cancer, rhBMP-2 use should be avoided.
YODA AND RHBMP-2 USAGE
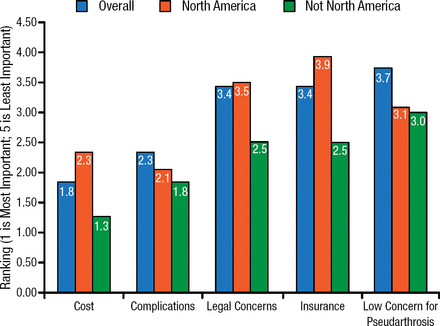
Scott D. Daffner, MD, West Virginia University, Morgantown, West Virgina, USA, presented survey results of about 800 practitioners regarding their use of rhBMP-2. He provided an overview of the results, showing that the most important reasons why clinicians did not use rhBMP-2 included legal concerns, insurance, and a low concern for pseudarthrosis (Figure 2).
Reasons Practitioners Do Not Use rhBMP-2
rhBMP-2, recombinant human bone morphogenetic protein-2. Reproduced with permission from WK Hsu, MD.
He also explained that there was a plateau in BMP use at about 28.3% of spinal fusion procedures beginning in 2008, followed by a 5% decrease in cervical fusions [Mckie J et al. Global Spine J. 2014].
He concluded with an overview of data from North American Spine Society (NASS) members. This illustrated member concerns for different types of complications, with around 60% reporting high concerns regarding cervical swelling and around 80% reporting mild or no concerns regarding cancer and retrograde ejaculation. Additionally, it gave insight into usage patterns and suggested that very few NASS members (0.71% and 2.1% for 2013 and 2014, respectively) had increased rhBMP-2 usage recently.
INCIDENCE OF COMPLICATIONS FROM RHBMP-2
Finally, Michael D. Daubs, MD, University of Nevada, Las Vegas, Nevada, USA, presented data on changes in rhBMP-2 usage over the past 3 years. After reviewing examples of complications, he showed results indicating a great deal of variability in the range of values presented for complications associated with BMP [Mroz TE et al. Spine (Phila PA 1976). 2010]. This variability raises the question of whether complications were reported accurately, whether there was error, or whether there have been changes in the way that BMP is used. Alternatively, the actual incidence of complications may have changed, potentially due to changes in usage and technique.
There appear to have been changes in the reported rates of some complications, although Dr Daubs noted that there is a difference in rates from questionnaires vs the laboratory. These changes could reflect changes in reporting rates, reduced occurrence, or changes in actual rates of complications as clinicians have responded to concerns about potential risks of BMPs. He concluded that it is not clear which explanation or combination of explanations is responsible for the changed rates.
- © 2015 MD Conference Express®