Summary
Lung cancer is the number one cause of cancer deaths in both men and women in the United States and around the world. To provide an overview of current and future imaging options, 5 specialists highlighted new concepts in the staging, imaging, and management of lung cancer.
- respiratory cancers
- magnetic resonance imaging
- tomography
Lung cancer is the number one cause of cancer deaths in both men and women in the United States and around the world [American Cancer Society. http://www.cancer.org/cancer/lungcancer-non-smallcell/detailedguide/non-small-cell-lung-cancer-key-statistics. Accessed January 12, 2015]. To provide an overview of current and future imaging options, 5 specialists highlighted new concepts in the staging, imaging, and management of lung cancer.
Fergus V. Gleeson, MBBS, Churchill Hospital, Headington, Oxford, United Kingdom, discussed contemporary concepts in small-cell lung cancer (SCLC). SCLC accounts for up to 15% of bronchogenic carcinomas [National Cancer Institute. http://www.cancer.gov/cancertopics/pdq/treatment/small-cell-lung/healthprofessional/page1#_6_toc. Accessed January 12, 2015] and around 20% of all lung cancers [Riaz SP et al. Lung Cancer. 2012]; most cases are attributable to smoking [Kalemkerian GP et al. J Natl Compr Canc Netw. 2013]. SCLC is characterized by its rapid growth, high response rates to both chemotherapy and radiotherapy, and development of treatment resistance in patients with metastatic disease [Früh M. Ann Oncol. 2013]. Incidence rates, however, appear to be falling [National Cancer Institute. http://www.cancer.gov/cancertopics/pdq/treatment/small-cell-lung/healthprofessional/page1#_6_toc. Accessed January 12, 2015].
Patients commonly present with anorexia, cough, hemoptysis, pain, as well as abnormal chest radiograph or computed tomography (CT) images; about 90% have a hilar mass and bulky lymphadenopathy [Carter BW et al. Radiographics. 2014]. It is unusual to find a solitary pulmonary nodule, and metastases are most commonly identified in the bone, liver, adrenal glands, and brain. Imaging for SCLC includes contrast-enhanced CT of the chest and liver, contrast-enhanced brain magnetic resonance imaging (MRI), and 2-[fluorine-18]-fluoro-2-deoxy-D-glucose (18-F-FDG) positron emission tomography (PET)-CT.
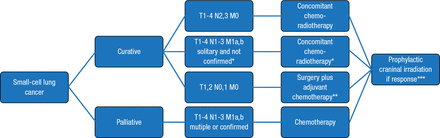
Management of localized disease (T1 to T4; N0 to 3; M0) is shown in Figure 1. Approximately 5% of patients with SCLC present with T1,2 N0,1 M0 tumors. These patients have more favorable survival outcomes over 5 years compared with patients with more severe disease [Schreiber D et al. Cancer. 2010; Yu JB et al. J Thorac Oncol. 2010].
European Society of Medical Oncology Clinical Practice Guidelines for Diagnosis, Treatment, and Follow-Up of Patients With Small-Cell Lung Cancer
*If no confirmation of solitary metastasis is obtained, radiotherapy may be added after first response evaluation and is omitted in case of obvious metastatic involvement.
**Concomitant chemoradiotherapy as an alternative option.
***Or stable disease in case of localised disease.
Reproduced from Früh M et al. Small-cell lung cancer (SCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24 (suppl 6):vi99–vi105. By permission of European Society for Medical Oncology.
Prognostic factors include the extent of disease, metabolic activity, paraneoplastic syndromes, elevated lactate dehydrogenase and creatinine, no histological or molecular features, and referral directly from a family doctor to a chest physician [Foster NR et al. Cancer. 2009]. Treatment options are surgery, radiotherapy, and chemotherapy [Powell HA et al. Br J Cancer. 2014]. The accuracy of staging is critical in the management of SCLC. Brain MRI should be performed at presentation, and surgery should be considered in patients with T1,2 and N0,1 disease.
NSCLC STAGING: CONCEPTS AND CONTROVERSIES
Ioannis Vlahos, MBBS, St George's Hospital NHS Trust, London, United Kingdom, highlighted the concepts and controversies associated with the staging of non–small cell lung cancer (NSCLC). The defined purpose of a staging system is to categorize disease severity, accurately predict prognosis, and guide patient management. The staging process should be easy to implement; should be logical, unambiguous, and reproducible; and should use all appropriate current imaging methodologies. The impetus for changing the TNM Classification of Malignant Tumors (TNM) staging system included evidence regarding technical improvement, such as the use of CT imaging and resection for NSCLC with intrapulmonary metastases.
The goals of the most recent (2007) TNM-7 staging system by the International Association for the Study of Lung Cancer were to reclassify TNM descriptors and staging groups according to survival [Goldstraw P et al. J Thorac Oncol. 2006]. The advantages of TNM-7 compared with TNM-6 include a large patient cohort (varied sources and treatments), clinical/CT-based staging, and a more accurate stratification of survival (Table 1).
Comparison of Data Sources Between Editions of the IASLC TNM
TNM-8, with expected publication in 2016, will address retrospective limitations, use a shorter data collection period (2009 to 2012), and make a series of changes for T, N, and M. It will also include histology, PET standard uptake values, comorbidities, and pulmonary function tests.
PET IMAGING IN NSCLC
Eric M. Rohren, MD, PhD, University of Texas MD Anderson Cancer Center, Houston, Texas, USA, discussed PET imaging of lung cancer. Although surgical resection remains the optimal treatment for early-stage NSCLC, approximately 40% of patients with stage I and 60% of those with stage II NSCLC relapse and die within 5 years of curative resection, indicating a need for additional prognostic biomarkers [Hyun SH et al. Ann Surg. 2013]. Dr Rohren reviewed published data that evaluated the prognostic significance and predictive performance of volume-based parameters of 18-F-FDG PET-CT in early-stage NSCLC. According to Hyun and colleagues [Ann Surg. 2013], the volume-based parameter of PET is an independent prognostic factor for survival and pathological TNM stage, and is a promising tool for better prediction of outcome in patients with early-stage NSCLC.
Dr Rohren also discussed non−18-F-FDG positron emission radiotracers for molecular imaging in oncology. These are used to measure glucose metabolism, cellular catabolism, receptors, membrane synthesis, proliferation, hypoxia, and bone turnover. New tracers under development and nearing regulatory approval in the United States include tracers targeting proliferation, receptor expression, and protein catabolism, as well as investigating molecular events and processes beyond glucose metabolism.
ADVANCES IN NODULE CHARACTERIZATION AND LUNG CANCER STAGING WITH MRI
Kyung Soo Lee, MD, Samsung Medical Center, Seoul, Korea, presented information about available MRI sequences for thoracic imaging; diffusion-weighted imaging (DWI) and its application in left coronary artery (LCA) imaging; and whole-body MRI and fusion imaging of MRI-PET in LCA staging.
Refined sequences in thoracic MRI include T2-weighted fast spin-echo for infiltrates and parenchymal lesions; T2-weighted fast spin-echo with fat saturated for lymph nodes (LNs) and bone lesions; T1W FS 3D-gradientecho for nodules and masses; steady-state free procession images for respiratory motion and lung vasculature; and DWI and fat saturated for nodule and LN characterization.
DWI can be used to perform single-shot echo-planar imaging and to measure microscopic water mobility (Brownian motion). Besides providing functional information, it can also be used to enhance image diffusion and process due to increased cellularity, fibrosis, and changes in intercellular spaces [Kwee TC et al. Eur Radiol. 2008; Nomori H et al. J Thorac Cardiovasc Surg. 2008; Ohno Y et al. Radiology. 2008].
Many advantages are associated with whole-body DWI with background body signal suppression. Multiple signal averaging, background body signal suppression by FS prepulse, and heavy DW in free-breathing state enable clinicians to highlight areas of restricted diffusion in primary malignancies and metastatic tumors, and visualize metastatic LNs [Kwee TC et al. Eur Radiol. 2008]. These images can be useful in cancer staging, response evaluation, and restaging.
CT PERFUSION IMAGING IN LUNG CANCER
Friedrich D. Knollmann, MD, University of California Davis Medical Center, Sacramento, California, USA, discussed the potential roles of computed tomography perfusion (CTP) imaging in tumor imaging, including the following:
-
Determination of tumor type, stage, and spread
-
Tumor detection
-
Treatment planning
-
Determination of treatment response
-
Restaging
-
Detection of tumor recurrence or metastatic disease
Although CTP is an attractive biomarker in lung cancer, there are challenges in using it to assess treatment response in lung cancer. These include anatomic coverage, respiratory motion, temporal resolution, determining an appropriate radiation dose, a dual blood supply of lung cancer, and the lack of standardized technique and analysis. Dr Knollmann gave an example of the difficulties in achieving anatomic coverage. This requires the following: a detector size of 2/4/16 cm; table motion that allows for axial and helical shuttle; table motion that reduces the sampling rate (sampling rates > 1 seconds influence perfusion parameters [Ng CS et al. AJR Am J Roentgenol. 2013]); a sampling interval that does not exceed 2 seconds [Miles KA et al. Eur Radiol. 2012]; and coverage of at least 4 cm.
He then discussed potential solutions to these problems, such as improved anatomic coverage with wider detectors and table motion, reduced radiation exposure with iterative reconstruction, advanced postprocessing with dual blood supply algorithms, motion registration and correction, and volumetric perfusion analysis.
THORACIC ONCOLOGIC IMAGING: TREATMENT EFFECTS AND COMPLICATIONS
Brett W. Carter, MD, University of Texas, Houston, Texas, USA, spoke about the role of imaging in patients who have been treated with radiation therapy, chemotherapy, or surgery for intrathoracic malignancies.
Among the surgical procedures for lung cancer—pneumonectomy, lobectomy, and sublobar resection—there are differences in the complication rate and risk of recurrence. Radiation therapy is typically one component of multimodality therapy for lung cancer, esophageal cancer, and lymphoma. Treatment plans are variable and depend on several factors.
The imaging appearance of irradiated tissue varies according to the time that has elapsed since the radiation. This can range from the ground-glass opacity of radiation pneumonitis (1 to 6 months) to the volume loss, consolidation, and traction bronchiectasis seen at 6 to 12 months. Patients who have undergone radiation are at risk of pulmonary embolism, esophagitis, ulceration, metastatic disease, hepatic injury, and radiation-induced sarcoma. Complications from chemotherapy include drug-induced pulmonary toxicity, pulmonary embolism, and arterial and venous thrombosis.
Dr Carter emphasized that CT and PET/CT are the modalities of choice in evaluating patients who have undergone surgery, radiation therapy, or chemotherapy. Knowledge of the spectrum of expected treatment-related changes, potential treatment complications, and how to identify tumor recurrence is critical in order to properly monitor patients, identify iatrogenic complications, and avoid misinterpretation.
- © 2015 MD Conference Express®