Summary
New imaging techniques and biomarkers are poised to improve the diagnosis of heart failure. The treatment of heart failure with preserved or reduced ejection fraction remains challenging. New drug treatments under investigation are natriuretic peptides, relaxin, ivabradine, spironolactone, and neprilysin. Stem cell therapy in heart failure is an active area of research.
- heart failure
- pulmonary edema
- nesiritide
- acute coronary syndrome
- relaxin
- preserved ejection fraction
- I-PRESERVE study
- brain natriuretic peptide
- stem cell therapy
- STEMI, myocardial infarction
- metaiodobenzylguanidine
- cardiology & cardiovascular medicine clinical trials
The treatment of heart failure (HF) is challenged by the limited therapies available, and there are currently no treatments that delay disease progression. Current treatments focus on relieving symptoms of HF, including pulmonary edema, which causes congestion and a feeling of breathlessness. Petar M. Seferović, MD, PhD, Medical School University of Belgrade, Belgrade, Serbia, discussed therapeutic possibilities for acute HF. Importantly, acute HF is associated with high mortality, not only in the hospital [Adams KF Jr et al. Am Heart J. 2005] but also for days and years after the initial event [McMurray JJ, Pfeffer MA. Lancet. 2005].
A mainstay in the initial treatment of acute HF are diuretics [Costanzo MR et al. Am Heart J. 2007], which help to relieve the congestion and breathlessness that most patients with acute HF experience. The 2012 European Society of Cardiology (ESC) guidelines recommend an intravenous loop diuretic to relieve congestion and breathlessness, with regular monitoring of symptoms, urine output, electrolytes, and renal function [McMurray JJV et al. Eur J Heart Fail. 2012]. Recent lessons learned from the failure of notable novel therapies to improve outcomes may position other new therapies on the horizon to better improve congestion and breathlessness without affecting renal function compared with traditional care.
Nesiritide is a vasodilator indicated for pulmonary congestion and edema in patients who have a systolic blood pressure > 90 mm Hg, because its primary adverse effect is hypotension. However, a meta-analysis of the NSGET, VMAC, and PROACTION trials demonstrated that treatment with nesiritide resulted in an increase in mortality throughout 30 days (adjusted HR, 1.80; 95% CI, 0.98 to 3.31; P = .057) [Sackner-Bernstein JD et al. JAMA. 2005]. As a result, nesiritide is considered to be a failed agent for the treatment of HF. Other failed agents include milrinone, tolvaptan, tezosentan, levosimendan, and adenosine antagonists.
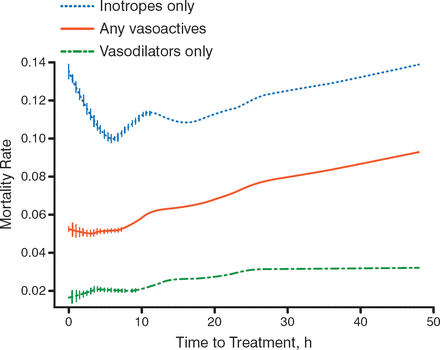
Several unsuccessful acute HF trials raised the question of changing the approach for the treatment of acute HF. Prof Seferović stated that acute HF should be approached in the same way that acute coronary syndrome (ACS) is approached—treatment, including vascular therapy, should be administered as early as possible. Several trials stressed that vasodilators may be the drug of choice. For example, in data from the ADHERE registry of more than 35 000 patients with acute HF, the adjusted odds of death rose by 6.8% for every 6-hour delay in treatment (95% CI, 4.2 to 9.6; P < .0001) [Peacock WF et al. Congest Heart Fail. 2009]. More specifically, early vasodilator treatment (1.7 hours) versus late vasodilator treatment (14.7 hours) was associated with a decreased rate of mortality (Figure 1). It is thought that because cardiac dilatation increases mortality through injury and loss of myocardium, early vasodilator therapy that decreases acute early cardiac dilatation will be beneficial.
Effect of Timing of Vasodilator Treatment in Acute Heart Failure
Adapted from Peacock WF et al. Early Vasoactive Drugs Improve Heart Failure Outcomes. Congest Heart Fail. 2009:15:256–264. With permission from John Wiley & Sons.
A new therapeutic approach is to use natriuretic peptides, such as urodilatin, which inhibits the reabsorption of sodium in the thick ascending limb of the kidney. In the SIRIUS-II trial [Mitrovic V et al. Eur Heart J. 2006], patients who received ularitide experienced lower rates of mortality, cardiac filling pressures, and serious adverse events, as well as improved dyspnea, compared with patients who received placebo. The randomized, phase 3, TRUE-AHF trial [NCT01661634] will further evaluate ularitide in patients with acute HF, with the primary end point of freedom from cardiovascular mortality, and a composite score including patient global assessment, lack of improvement or worsening of HF that requires intervention, and death.
Another emerging therapy is relaxin, a hormone that has increased secretion during pregnancy, mediates hemodynamic changes, and has anti-ischemic, anti-inflammatory, and anti-fibrotic functions in pregnant women [Helal I et al. Nat Rev Nephrol. 2012]. The dose-finding, phase 2, Pre-RELAX-AHF study [Teerlink JR et al. Lancet. 2009] demonstrated that relaxin treatment resulted in trends of improved dyspnea and congestion, reduced need for diuretic therapy, and reduced worsening of HF, as well as a significant decrease in cardiovascular death throughout 180 days compared with placebo. No significant adverse events or serious hypotensive events were reported. However, HF-associated rehospitalization was similar among the serelaxin and placebo arms.
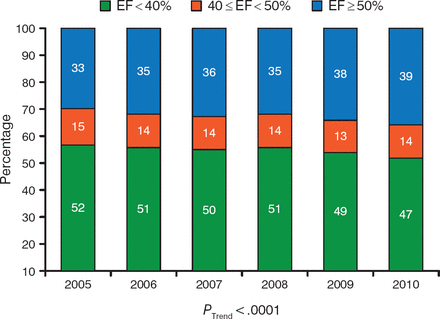
During the past decade, the prevalence of heart failure with preserved ejection fraction (HFpEF) has steadily increased [Owan TE et al. N Engl J Med. 2006], as has the number of hospital admissions of patients with HFpEF (Figure 2) [Steinberg BA et al. Circulation. 2012]. In addition, patients who are hospitalized for HFpEF demonstrated similar rates of postdischarge mortality and rehospitalization for ≤ 90 days as patients with heart failure with reduced ejection fraction (HFrEF) [Fonarow GC et al. J Am Coll Cardiol. 2007]. Burkert Pieske, MD, Medical University of Graz, Graz, Austria, presented updates in the diagnosis and treatment of HFpEF.
Proportion of Patients Hospitalized With Reduced and Preserved Ejection Fraction
EF, ejection fraction.
Adapted from Steinberg BA et al. Health Services and Outcomes Research: Trends in Patients Hospitalized With Heart Failure and Preserved Left Ventricular Ejection Fraction. Circulation. 2012;126:65–75. With permission from American Heart Association.
The diagnosis of HFpEF can be difficult. A subanalysis of the I-PRESERVE study demonstrated that in almost 50% of patients with HFpEF, there was no structural remodeling of the left ventricle [Zile MR et al. Circulation. 2011]. The Heart Failure and Echocardiography Associations of the ESC recommend in their guideline that the diagnosis of HFpEF be made based on signs and symptoms of HF; preserved systolic left ventricular (LV) function with an ejection fraction (EF) > 50%; evidence of abnormal LV relaxation, filling, compliance, or stiffness; and increased BNP or NT-proBNP [Paulus WJ et al. Eur Heart J. 2007].
In addition, new echocardiography (ECHO) techniques and parameters and new biomarkers can help clinicians diagnose HFpEF. For example, the exercise ECHO test, or diastolic stress test, can demonstrate the abnormal hemodynamics of patients with HFpEF [Borlaug BA, Paulus WJ. Eur Heart J. 2011].
Unfortunately, there appears to be little improvement in the advancement of new therapies for HFpEF. A meta-analysis of several large trials in HFpEF demonstrated that there was no clear benefit of HFrEF therapies for patients with HFpEF [Redfield MM et al. Circ Heart Fail. 2012]. However, there are several emerging therapies in the pipeline that target various aspects of HFpEF.
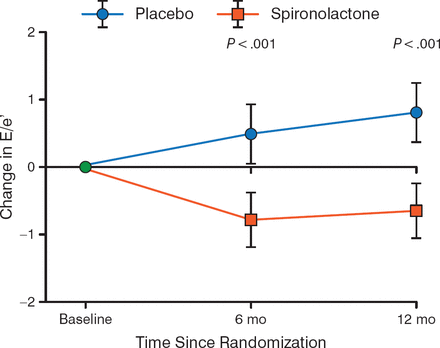
Ivabradine may improve heart rate in patients with HFpEF, because one study demonstrated that peak oxygen volume was greater in patients who received ivabradine compared with placebo [Kosmala W et al. J Am Coll Cardiol. 2013]. The randomized Aldo-DHF trial demonstrated that treatment of HFpEF with spironolactone resulted in a decreased change in diastolic function on ECHO compared with placebo throughout 12 months (P < .001; Figure 3) [Edelmann F et al. JAMA. 2013].
Effect of Spironolactone on Diastolic Function in HFpEF
E/e', early transmitral ventricular filling velocity to early diastolic tissue Doppler velocity; HFpEF, heart failure with preserved ejection fraction.
Adapted from Edelmann F et al. Effect of Spironolactone on Diastolic Function and Exercise Capacity in Patients With Heart Failure With Preserved Ejection Fraction: The Aldo-DHF Randomized Controlled Trial. JAMA 2013;309:781–791. Copyright © 2013 American Medical Association. All rights reserved.
In addition, data from the TOPCAT trial [Pitt B et al. N Engl J Med. 2014] demonstrated reduced hospitalization rates for patients with HFpEF who received spironolactone compared with placebo (P = .04), but the primary composite outcome of cardiovascular death, HF-related hospitalization, or resuscitated cardiac arrest was similar between both arms. The results of this trial were marred by the dramatic difference in response to the placebo according to geographic area; the primary composite outcome was achieved by 12.6 per 100 patient-years in patients from North and South America, whereas only 2.3 per 100 patient-years achieved the primary outcome in Russia and the Republic of Georgia.
Another emerging therapy is inhibition of the angiotensin receptor neprilysin via LCZ696. The phase 2 PARAMOUNT trial [Solomon SD et al. Lancet. 2012] demonstrated that treatment with LCZ696 resulted in a decrease in NT-proBNP compared with valsartan throughout 36 weeks, with significance reached at 12 weeks (P = .005). Another approach to the treatment of HFpEF targets cyclic guanylate monophosphate production by stimulating soluble guanylate cyclase (sGC). In the DILATE study [Bonderman D et al. ESC 2013 (poster P3321)], single doses of riociguat resulted in increased cardiac output in a dose–response fashion. As a result, the SOCRATES program [Pieske B et al. Eur J Heart Fail. 2014] was initiated, which includes 2 randomized trials that will evaluate the effect of the sGC stimulator vericiguat throughout 12 weeks in patients with worsening chronic HF; the SOCRATES-REDUCED trial will enroll patients with a reduced EF; and the SOCRATES-PRESERVED trial will enroll patients with a preserved EF.
Stem cell therapy represents a completely new approach in the treatment of H F. Francisco Fdez-Avilés, MD, PhD, Hospital General Universitario Gregorio Marañón, Madrid, Spain, provided an overview of stem cell therapy for both the prevention and treatment of H F. After clear evidence of stem cell cardiac regeneration in humans and positive results with embryonic and adult stem cells in animal models, stem cell therapy was first evaluated in ischemic heart disease in the setting of STEMI with or without reduced left ventricular ejection fraction (LVEF). In a meta-analysis of 50 randomized controlled trials, bone marrow–derived cells administered via a catheter to > 2600 patients resulted in improved LV and clinical outcomes without causing short- or long-term complications [Jeevanantham V et al. Circulation. 2012]. In addition, the REPAIR-AMI trial [NCT00279175] demonstrated an increase in LVEF compared with placebo, as well as with stent plus abciximab (relative comparison). Stem cells harvested from other tissue, including cardiac, adipose, or muscle, have also demonstrated promising results in ischemic heart disease.
Although cardiac stem cells demonstrated promising results in 2 small studies in patients with reduced LVEF [Makkar RR et al. Lancet. 2012; Bolli R et al. Lancet. 2011], a framework or scaffolding was needed to aid in the organization of the growing cells and to provide perfusion. As a result, the SABIO project, which has been granted by the Spanish government and is being carried out with the collaboration of the Texas Heart Institute and 2 Spanish institutions (University Hospital Gregorio Maranon and Spanish National Transplant Organization), will evaluate scaffolds and bioartificial organs for transplantation. This process harvests autologous stem cells from the recipient, which will be applied to a 3D scaffold built from a cadaveric heart and eventually transplanted into the recipient.
Current research is expanding the application of stem cell therapy in other cardiac conditions, including arrhythmias, cardiomyopathy, and pulmonary hypertension. In addition, new delivery methods are being assessed.
Advances in imaging tests can improve the diagnosis of HF, enabling patients to receive earlier treatment for their condition. Randall C. Thompson, MD, Saint Luke's Mid America Heart Institute, Kansas City, Missouri, USA, discussed the use of metaiodobenzylguanidine (MIBG) in HF. MIBG imaging uses the heart-to-mediastinum (H/M) ratio to predict survival in patients with HF, in which a very low H/M ratio (< 1.20) is associated with the lowest survival rate.
MIBG imaging can be used to assess patients with arrhythmias, which cause an estimated 184 000 to 462 000 cardiovascular deaths annually in the United States [Goldberger JJ et al. J Am Coll Cardiol. 2008]. In particular, MIBG imaging may identify patients who are at risk of sudden cardiac death, enabling clinicians to recommend implantable cardiac defibrillators to patients who would benefit the most.
When performing MIBG imaging, the ADMIRE-HF study [Jacobson AF et al. J Am Coll Cardiol. 2010] demonstrated that using the entire heart as the region of interest (ROI) resulted in greater consistency in ROI and allows for the best estimate of average uptake of MIBG. In addition, the ROI of the mediastinum should be centered along the midvertical, upper mediastinum. Using these ROIs, the H/M is equal to the counts per pixel in the cardiac ROI versus the counts per pixel of the mediastinal ROI. To determine the washout rate of 123I-mIBG, the H/M ratio of the delayed image is subtracted from the H/M ratio of the early image, divided by the H/M ratio of the early image, multiplied by 100.
Prior to performing MIBG imaging, it is important to determine if the patient is taking any drugs that may interfere with MIBG neuronal accumulation, such as tricyclic antidepressants, calcium antagonists, and sympathicomimetic amines. In the case when a patient is taking such an agent, the agent must be discontinued for 5 biological half-lives prior to the MIBG imaging test [AdreView (package insert). Arlington Heights, IL: GE Healthcare; 2008]. In the cardiac MIBG report, clinicians should include patient details, clinical indication for the test, appearance of the images and their quality, planar images, the H/M ratio, washout, sympathetic activity defects, other pertinent findings such as LV dilatation or E F, and any potential artifacts [Flotats A et al. Eur J Nucl Med Mol Imaging. 2010].
Although advances in the treatment of HF with both reduced and preserved EF have been slow, there are emerging agents on the horizon for both conditions. In addition, new techniques in imaging and biomarkers will improve the diagnosis of HF, enabling patients to receive treatment earlier.
- © 2015 SAGE Publications