Summary
Identifying viable myocardium with FDG-PET imaging identified patients with left ventricular (LV) dysfunction who benefited from revascularization and had improved survival. Contrast-enhanced magnetic resonance imaging also has been shown to identify viable myocardium. Baseline ejection fraction, magnitude of myocardial scarring, degree of LV remodeling, and time to revascularization are inversely related to functional recovery after revascularization.
- left ventricular dysfunction
- surgical revascularization
- coronary artery bypass grafting
- F-18-fluorodeoxyglucose
- positron emission tomography
- magnetic resonance imaging
- revascularization
- heart failure
- coronary artery disease
- imaging modalities
Up to 61% of patients with left ventricular (LV) dysfunction still have some viable myocardium. Ahmad Fathala, MD, King Faisal Specialist Hospital and Research Center, Riyadh, Saudi Arabia, believes that revascularization should continue even in the face of jeopardized—but still viable—myocardium.
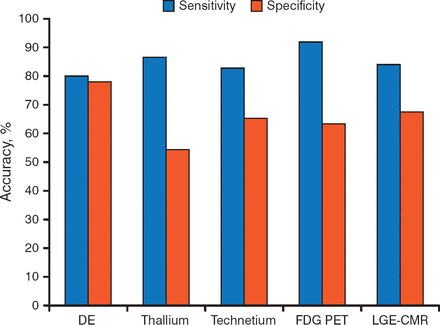
Surgical revascularization may improve heart failure (HF) symptoms, LV ejection fraction (EF), and long-term prognosis in patients with coronary artery disease (CAD) who have a substantial amount of viable myocardium. A variety of methods can be used to identify patients with viable myocardium and to predict patient outcomes, but the sensitivity and specificity vary among the techniques (Figure 1) [Schinkel AF et al. Curr Probl Cardiol. 2007].
Sensitivity and Specificity of Various Imaging Methods for Identifying Myocardial Viability
DE, dobutamine echocardiography; FDG PET, F-18 fluorodeoxyglucose positron-emission tomography; LGE-CMR, late gadolinium enhancement cardiovascular magnetic resonance.
Source: Schinkel AF et al. Curr Probl Cardiol. 2007. Reproduced with permission from A Fathala, MD.
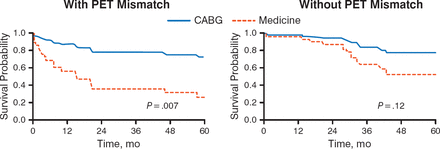
In 1998, a study was conducted to assess the long-term benefit of myocardial viability assessment for stratifying risk and selecting patients with low EF for coronary artery bypass grafting (CABG). It found that patients with severe CAD, low EF (median, 25%), and evidence of viable myocardium on positron-emission tomography (PET) had significantly improved 4-year survival (75% vs 30%; P = .007; Figure 2). After CABG, patients also had significant improvement in angina and HF symptoms compared with those receiving medical therapy.
Prognosis of Patients With LV Dysfunction by PET Pattern of Viability and Mode of Treatment
CABG, coronary artery bypass grafting; LV, left ventricular; PET, positron-emission tomography.
Adapted from the Journal of Thoracic and Cardiovascular Surgery, 116, Di Carli MF et al, Long-term survival of patients with coronary artery disease and left ventricular dysfunction: Implications for the role of myocardial viability assessment in management decisions, 997–1004, Copyright (1998), with permission from Mosby, Inc.
An important question is whether patient management based on results of a myocardium viability assessment makes a difference in outcome. Beanlands and colleagues attempted to answer this question in a study that assessed myocardial viability through F-18 fluorodeoxyglucose (FDG) PET. The results showed no difference in the primary outcome—a composite of cardiac death, myocardial infarction, or recurrent hospital stay for cardiac cause, within 1 year—for patients assigned to management based on FDG PET versus standard care. Actual compliance with the PET-recommended treatment was poor (25% of patients with PET-indicated revascularization did not have it done). When the data were reanalyzed to include only patients receiving PET-adherent treatment, a significant survival benefit was seen (P = .019) [Beanlands RS et al. J Am Coll Cardiol. 2007]. This survival benefit associated with FDG PET management was recently confirmed [Abraham A et al. J Nucl Med. 2010].
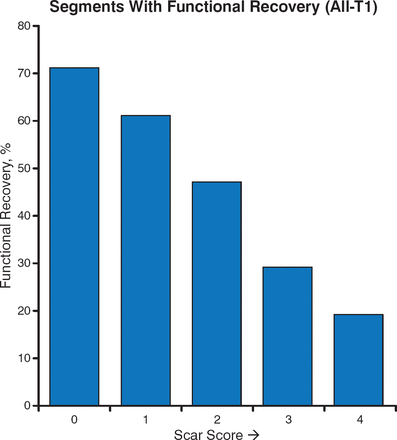
Magnetic resonance imaging (MRI) is also useful in assessing myocardial viability. Contrast-enhanced MRI with late gadolinium enhancement can identify reversible or stunning myocardial dysfunction and determine if regions of abnormal ventricular contraction will improve after revascularization in patients with CAD (Figure 3) [Kim RJ et al. N Engl J Med. 2000].
LGE Identifies Reversible Myocardial Dysfunction Prior to Revascularization
LGE, late gadolinium enhancement.
Source: Kim RJ et al. N Engl J Med. 2000. Reproduced with permission from A Fathala, MD.
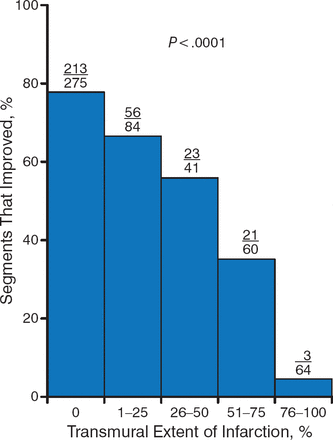
Delayed-enhancement MRI is a robust predictor of functional recovery after revascularization. Results of a prospective international multicenter trial showed an inverse relationship between the extent of myocardial scarring, as determined by contrast MRI, and functional recovery. The higher the scar score, the fewer myocardial segments that recovered after revascularization (Figure 4) [Lenge VV et al. J Cardiovasc Mag Res. 2008]. In another trial that assessed the prognostic significance of unrecognized myocardial scarring in patients with no history of myocardial infarction, the presence and extent of myocardial scarring as detected by cardiovascular magnetic resonance was a strong predictor of major adverse cardiac events and cardiac death [Kwong RY et al. Circulation. 2006].
Inverse Relationship Between Extent of Scar and Functional Recovery
Adapted from Lenge VV et al. 124 Delayed-enhancement MRI as a predictor of functional recovery after revascularization: results from an International Multicenter Viability Trial. J Cardiovasc Mag Res. 2008;10:A25. With permission from Lenge VV et al and BioMed Central.
Factors inversely related to recovery of function after revascularization include baseline EF, magnitude of myocardial scarring, degree of LV remodeling, and time to revascularization. Among the techniques used to detect myocardial viability, nuclear techniques are the most sensitive, while dobutamine echo cardiography has the highest specificity. MRI can detect scar tissue, but it does not provide any information on nonscar tissue. Prof Fathala concluded that from a practical point of view, clinicians should proceed with revascularization once viable myocardium is detected.
- © 2015 SAGE Publications