Summary
The new bioresorbable drug-eluting stents are deliverable, usable at bifurcations, compatible with short-term dual antiplatelet therapy, and low cost. Long-term outcomes are needed, however, to confirm their safety and efficacy compared with permanent drug-eluting stents.
- coronary artery disease
- stent
- drug-eluting stents
- bare metal stents
- target lesion revascularization
- stent thrombosis
- cardiology & cardiovascular medicine clinical trials
- percutaneous coronary intervention
Patients with coronary artery disease (CAD) undergoing percutaneous revascularization are treated with either drug-eluting stents (DESs) or bare metal stents. Although new generations of DESs have been developed, these stents continue to have some limitations. Bernard Chevalier, MD, Institut Cardiovasculaire Paris Sud, Massy, France, presented current data on the bioresorbable DESs that are in development.
First-generation, polymer-based DESs had multiple limitations. The polymer was fragile, resulting in uneven drug distribution that increased the risk of focal instent restenosis. The kinetics of drug release were not consistent and increased the risk of diffuse restenosis. Some stents had prolonged elution of the medications designed to prevent restenosis that delayed endothelialization of the stents and increased the risk of stent thrombosis. Subsequent generations of DESs have sought to eliminate these issues.
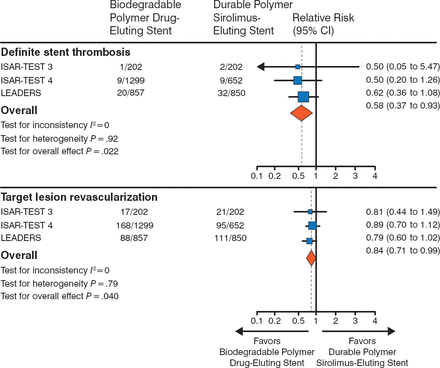
DESs with biodegradable polymers are under development in an effort to avoid long-term inflammation and improve clinical outcomes. In the LEADERS trial [Stefanini GG et al. Lancet. 2011], patients with CAD were randomized to either a biodegradable biolimus-eluting stent (BES) or a durable polymer sirolimus-eluting stent (SES) and were followed for 4 years. The biodegradable BES was noninferior to the durable polymer SES for the end points of target lesion revascularization (TLR) and definite stent thrombosis (Figure 1).
Biodegradable Vs Durable Polymer Drug-Eluting Stents
Adapted from The Lancet, 378, Stefanini GG et al. Long-term clinical outcomes of biodegradable polymer biolimus-eluting stents versus durable polymer sirolimus-eluting stents in patients with coronary artery disease (LEADERS): 4 year follow-up of a randomised non-inferiority trial. 1940–1948. Copyright © 2011, with permission from Elsevier.
The NEXT trial [Natsuaki M et al. J Am Coll Cardiol. 2013] demonstrated that TLR and stent thrombosis occurred at similar, but very low, rates among patients who received the biodegradable BES compared with the durable polymer SES.
The CENTURY II trial [Saito S et al. Eur Heart J. 2014] randomized patients to either the bioresorbable Ultimaster SES or the permanent Xience everolimus-eluting stent. The Ultimaster stent is made of a PDLLA-PCL copolymer that is resorbed within 3 to 4 months. By contrast, the Xience DES contains a PVDF-HFP nonerodible fluorinated copolymer and is permanent. Patients treated with the Ultimaster stent had a 0.40% higher rate of freedom from target lesion failure (95% CI, −2.22 to 3.02; P = .0001) at 9 months. In the cohort of patients from Japan, the cumulative incidence of TLR events was 4.14% (95% CI, 2.52 to 6.78) and 5.67% (95% CI, 3.69 to 8.64) in the Xience arm.
A variety of other bioresorbable stents, including both polymer- and nonpolymer-based stents, and stents that elute everolimus, novolimus, and sirolimus, have undergone animal and clinical trials. To date, none of the bioresorbable stents are approved for use in the United States. In Europe, only Abbott's BVS 1.1 polymer-based stent is available for clinical use.
Prof Chevalier described clinical challenges that might arise when deploying bioresorbable stents. Bioresorbable stents are susceptible to mechanical deformation of stent strut with delivery. In addition, as the stent polymer erodes, the radial strength of the polymer decreases, which could result in late lumen loss. The ABSORB II trial [Diletti R et al. Am Heart J. 2012] randomized patients in a 2:1 fashion to receive the Absorb bioresorbable stent or the Xience stent. The primary end points of the study are based on changes in lumen diameter.
Prof Chevalier highlighted that the current limitations of the bioresorbable DES include a large profile (> 1.4 mm), decreased radial strength over time, and limited ability to increase the diameter using postdilation inflations. The potential benefits of a bioabsorbable stent would not begin until after the PCI (eg, strut resorption, conformability, pulsatility, vasomotoricity, plaque regression, and positive remodeling). He cautioned that bioresorbable DESs have been evaluated in a relatively small number of patients and have only been used to treat simple lesions.
Current work is focused on developing thinner stent struts with a lower profile in an attempt to reduce occlusion of small side branches. Other areas of work include increasing the ability the stents to be sized further after deployment and the development new polymers and strut designs. In the meantime, Prof Chevalier pointed out that lesion preparation and appropriate sizing are important prior to deployment of a stent, particularly in bioresorbable stents, in order to prevent mechanical deformation and to achieve the best possible outcomes.
In conclusion, Prof Chevalier stated that the new, bioresorbable stents are deliverable, can be used at bifurcations, are cost-effective, and are compatible with short-term dual antiplatelet therapy. Current data suggest that bioresorbable DESs have similar short-term efficacy and safety as permanent DESs but data on long-term outcomes are needed.
- © 2015 SAGE Publications